The Potential for Renal Injury Elicited by Physical Work in the Heat
- PMID: 31487794
- PMCID: PMC6769672
- DOI: 10.3390/nu11092087
The Potential for Renal Injury Elicited by Physical Work in the Heat
Abstract
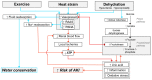
An epidemic of chronic kidney disease (CKD) is occurring in laborers who undertake physical work in hot conditions. Rodent data indicate that heat exposure causes kidney injury, and when this injury is regularly repeated it can elicit CKD. Studies in humans demonstrate that a single bout of exercise in the heat increases biomarkers of acute kidney injury (AKI). Elevations in AKI biomarkers in this context likely reflect an increased susceptibility of the kidneys to AKI. Data largely derived from animal models indicate that the mechanism(s) by which exercise in the heat may increase the risk of AKI is multifactorial. For instance, heat-related reductions in renal blood flow may provoke heterogenous intrarenal blood flow. This can promote localized ischemia, hypoxemia and ATP depletion in renal tubular cells, which could be exacerbated by increased sodium reabsorption. Heightened fructokinase pathway activity likely exacerbates ATP depletion occurring secondary to intrarenal fructose production and hyperuricemia. Collectively, these responses can promote inflammation and oxidative stress, thereby increasing the risk of AKI. Equivalent mechanistic evidence in humans is lacking. Such an understanding could inform the development of countermeasures to safeguard the renal health of laborers who regularly engage in physical work in hot environments.
Keywords: acute kidney injury; chronic kidney disease; dehydration; exercise; heat stress.
Conflict of interest statement
The authors have no related conflicts of interest to report.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

