Multi-Staged Regulation of Lipid Signaling Mediators during Myogenesis by COX-1/2 Pathways
- PMID: 31487817
- PMCID: PMC6769623
- DOI: 10.3390/ijms20184326
Multi-Staged Regulation of Lipid Signaling Mediators during Myogenesis by COX-1/2 Pathways
Abstract
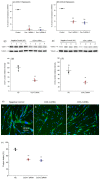
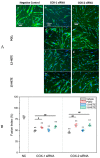
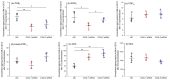
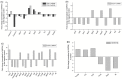
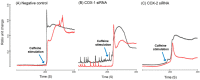
Cyclooxygenases (COXs), including COX-1 and -2, are enzymes essential for lipid mediator (LMs) syntheses from arachidonic acid (AA), such as prostaglandins (PGs). Furthermore, COXs could interplay with other enzymes such as lipoxygenases (LOXs) and cytochrome P450s (CYPs) to regulate the signaling of LMs. In this study, to comprehensively analyze the function of COX-1 and -2 in regulating the signaling of bioactive LMs in skeletal muscle, mouse primary myoblasts and C2C12 cells were transfected with specific COX-1 and -2 siRNAs, followed by targeted lipidomic analysis and customized quantitative PCR gene array analysis. Knocking down COXs, particularly COX-1, significantly reduced the release of PGs from muscle cells, especially PGE2 and PGF2α, as well as oleoylethanolamide (OEA) and arachidonoylethanolamine (AEA). Moreover, COXs could interplay with LOXs to regulate the signaling of hydroxyeicosatetraenoic acids (HETEs). The changes in LMs are associated with the expression of genes, such as Itrp1 (calcium signaling) and Myh7 (myogenic differentiation), in skeletal muscle. In conclusion, both COX-1 and -2 contribute to LMs production during myogenesis in vitro, and COXs could interact with LOXs during this process. These interactions and the fine-tuning of the levels of these LMs are most likely important for skeletal muscle myogenesis, and potentially, muscle repair and regeneration.
Keywords: Cyclooxygenase; lipidomics; myogenic differentiation; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Catalytically active phospholipase A2 myotoxin from Crotalus durissus terrificus induces proliferation and differentiation of myoblasts dependent on prostaglandins produced by both COX-1 and COX-2 pathways.Int J Biol Macromol. 2021 Sep 30;187:603-613. doi: 10.1016/j.ijbiomac.2021.07.121. Epub 2021 Jul 25. Int J Biol Macromol. 2021. PMID: 34314795
-
Feline calicivirus- and murine norovirus-induced COX-2/PGE2 signaling pathway has proviral effects.PLoS One. 2018 Jul 18;13(7):e0200726. doi: 10.1371/journal.pone.0200726. eCollection 2018. PLoS One. 2018. PMID: 30021004 Free PMC article.
-
Exploration of binding site pattern in arachidonic acid metabolizing enzymes, Cyclooxygenases and Lipoxygenases.BMC Res Notes. 2015 Apr 16;8:152. doi: 10.1186/s13104-015-1101-4. BMC Res Notes. 2015. PMID: 25886468 Free PMC article.
-
[Cyclooxygenases, lipoxygenases, their targeted drugs and the prevention of Alzheimer's disease].Yao Xue Xue Bao. 2013 Dec;48(12):1743-54. Yao Xue Xue Bao. 2013. PMID: 24689230 Review. Chinese.
-
Pathophysiological Roles of Cyclooxygenases and Prostaglandins in the Central Nervous System.Mol Neurobiol. 2016 Sep;53(7):4754-71. doi: 10.1007/s12035-015-9355-3. Epub 2015 Sep 2. Mol Neurobiol. 2016. PMID: 26328537 Review.
Cited by
-
Effect of nonsteroidal anti-inflammatory drugs on pelvic floor muscle regeneration in a preclinical birth injury rat model.Am J Obstet Gynecol. 2024 Apr;230(4):432.e1-432.e14. doi: 10.1016/j.ajog.2023.12.001. Epub 2023 Dec 6. Am J Obstet Gynecol. 2024. PMID: 38065378 Free PMC article.
-
A simple model of immune and muscle cell crosstalk during muscle regeneration.Math Biosci. 2021 Mar;333:108543. doi: 10.1016/j.mbs.2021.108543. Epub 2021 Jan 16. Math Biosci. 2021. PMID: 33465385 Free PMC article.
-
Market Needs and Methodologies Associated with Patient Lipidomic Diagnoses and Analyses.Methods Mol Biol. 2024;2816:53-67. doi: 10.1007/978-1-0716-3902-3_6. Methods Mol Biol. 2024. PMID: 38977588 Free PMC article.
-
Numb is required for optimal contraction of skeletal muscle.J Cachexia Sarcopenia Muscle. 2022 Feb;13(1):454-466. doi: 10.1002/jcsm.12907. Epub 2022 Jan 9. J Cachexia Sarcopenia Muscle. 2022. PMID: 35001540 Free PMC article.
-
Pressing Intervention Promotes the Skeletal Muscle Repair of Traumatic Myofascial Trigger Points in Rats.J Pain Res. 2021 Oct 15;14:3267-3278. doi: 10.2147/JPR.S333705. eCollection 2021. J Pain Res. 2021. PMID: 34703302 Free PMC article.
References
-
- Karalaki M., Fili S., Philippou A., Koutsilieris M. Muscle regeneration: Cellular and molecular events. In Vivo. 2009;23:779–796. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

