A Comprehensive Examination of Percutaneous Endoscopic Gastrostomy and Its Association with Amyotrophic Lateral Sclerosis Patient Outcomes
- PMID: 31487846
- PMCID: PMC6770872
- DOI: 10.3390/brainsci9090223
A Comprehensive Examination of Percutaneous Endoscopic Gastrostomy and Its Association with Amyotrophic Lateral Sclerosis Patient Outcomes
Abstract
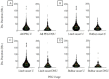
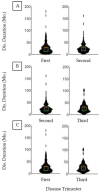
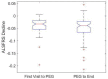
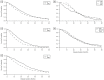
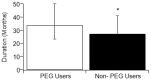
There is literature discord regarding the impact of percutaneous endoscopic gastrostomy (PEG), or "feeding tube", on amyotrophic lateral sclerosis (ALS) outcomes. We assess one of the largest retrospective ALS cohorts to date (278 PEG users, 679 non-users). Kruskal-Wallis and Kaplan-Meier analysis compared cohort medians and survival duration trends. A meta-analysis determined the aggregate associative effect of PEG on survival duration by combining primary results with 7 published studies. Primary results (p < 0.001) and meta-analysis (p < 0.05) showed PEG usage is associated with an overall significant increase in ALS survival duration, regardless of onset type. Percent predicted forced vital capacity (FVC %predict) ≥50 at PEG insertion significantly increases survival duration (p < 0.001); FVC %predict ≥60 has the largest associative benefit (+6.7 months, p < 0.05). Time elapsed from ALS onset until PEG placement is not predictive (p > 0.05). ALSFRS-R survey assessment illustrates PEG usage does not slow functional ALS pathology (p > 0.05), but does stabilize weight and/or body mass index (BMI) (p < 0.05). Observed clinical impression of mood (CIM), was not impacted by PEG usage (p > 0.05). Overall results support PEG as a palliative intervention for ALS patients with ≥50 FVC %predict at PEG insertion.
Keywords: ALS; dysphagia; malnutrition; motor neuron disease.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Czell D., Bauer M., Binek J., Schoch O.D., Weber M. Outcomes of percutaneous endoscopic gastrostomy tube insertion in respiratory impaired amyotrophic lateral sclerosis patients under noninvasive ventilation. Respir. Care. 2013;58:838–844. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

