Selenium, Selenoproteins and Viral Infection
- PMID: 31487871
- PMCID: PMC6769590
- DOI: 10.3390/nu11092101
Selenium, Selenoproteins and Viral Infection
Abstract
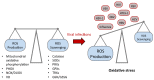
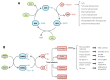
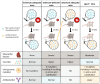
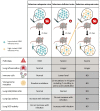
Reactive oxygen species (ROS) are frequently produced during viral infections. Generation of these ROS can be both beneficial and detrimental for many cellular functions. When overwhelming the antioxidant defense system, the excess of ROS induces oxidative stress. Viral infections lead to diseases characterized by a broad spectrum of clinical symptoms, with oxidative stress being one of their hallmarks. In many cases, ROS can, in turn, enhance viral replication leading to an amplification loop. Another important parameter for viral replication and pathogenicity is the nutritional status of the host. Viral infection simultaneously increases the demand for micronutrients and causes their loss, which leads to a deficiency that can be compensated by micronutrient supplementation. Among the nutrients implicated in viral infection, selenium (Se) has an important role in antioxidant defense, redox signaling and redox homeostasis. Most of biological activities of selenium is performed through its incorporation as a rare amino acid selenocysteine in the essential family of selenoproteins. Selenium deficiency, which is the main regulator of selenoprotein expression, has been associated with the pathogenicity of several viruses. In addition, several selenoprotein members, including glutathione peroxidases (GPX), thioredoxin reductases (TXNRD) seemed important in different models of viral replication. Finally, the formal identification of viral selenoproteins in the genome of molluscum contagiosum and fowlpox viruses demonstrated the importance of selenoproteins in viral cycle.
Keywords: coxsackie virus; glutathione peroxidases; hepatitis C virus; human immunodeficiency virus; immunity; influenza virus; molluscum contagiosum virus; reactive oxygen species; thioredoxin reductases; viral selenoproteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hatfield D.L., Carlson B.A., Xu X.M., Mix H., Gladyshev V.N. Selenocysteine incorporation machinery and the role of selenoproteins in development and health. Prog. Nucleic Acid Res. Mol. Biol. 2006;81:97–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

