Role of Herpes Simplex Envelope Glycoprotein B and Toll-Like Receptor 2 in Ocular Inflammation: An ex vivo Organotypic Rabbit Corneal Model
- PMID: 31487910
- PMCID: PMC6783931
- DOI: 10.3390/v11090819
Role of Herpes Simplex Envelope Glycoprotein B and Toll-Like Receptor 2 in Ocular Inflammation: An ex vivo Organotypic Rabbit Corneal Model
Abstract
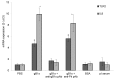
It has been recently reported, using in vitro studies, that the herpes simplex virus 1 (HSV-1) encoded envelope glycoprotein B (gB1) interacts with cell surface toll-like receptor 2 (TLR2) and induces the secretion of interleukin-8 (IL8), a representative marker of inflammatory cytokine activation. The purpose of this study is to investigate the role of gB1 in activating host inflammatory responses by using a secreted form of gB1 (gB1s) and an ex vivo organotypic rabbit corneal model. Abraded corneas exposed to gB1s alone or to the recombinant protein mixed with anti gB polyclonal antibody were cultured in an air-liquid interface. The corneas exposed to gB1s show the appearance of mydriasis and high levels of TLR2 and IL-8 mRNAs transcripts were detected in the superficial layer of corneal epithelial cells. Histological stain and immunohistochemical analyses revealed morphological changes in the epithelium of the treated corneas and variations in expression and localization of TLR2. Collectively these findings provide new insight into the pathogenesis of HSV-1 ocular infection by demonstrating the leading role of gB in activating an inflammatory response and in the appearance of mydriasis, a sign of HSV-1 anterior uveitis.
Keywords: HSV-1 gB; TLR2; ex vivo corneal model; mydriasis.
Conflict of interest statement
The authors declare no conflict of interest. The funder has no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

