Cyclobutane-Containing Scaffolds as Useful Intermediates in the Stereoselective Synthesis of Suitable Candidates for Biomedical Purposes: Surfactants, Gelators and Metal Cation Ligands
- PMID: 31487921
- PMCID: PMC6770955
- DOI: 10.3390/ijms20184333
Cyclobutane-Containing Scaffolds as Useful Intermediates in the Stereoselective Synthesis of Suitable Candidates for Biomedical Purposes: Surfactants, Gelators and Metal Cation Ligands
Abstract
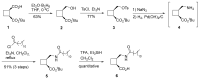
Efficient and versatile synthetic methodologies are reported for the preparation of products that are suitable candidates to be used as surfactants, gelators for hydroxylic solvents or metal cation ligands, with potential use in several fields including biomedical applications. The common structural feature of all the synthesized products is the presence of a cis or trans-1,2- or cis-1,3-difunctionalized cyclobutane ring. In the two first cases, the key intermediates including enantiomerically pure 1,3-diamines and 1,3-amino alcohols have been prepared from β-amino acid derivatives obtained, in turn, from a chiral half-ester. This compound is also precursor of γ-amino esters. Furthermore, two kind of polydentate ligands have also been synthesized from a symmetric 1,5-diamine obtained from norpinic acid, which was easily prepared from commercial verbenone.
Keywords: amphiphiles; cation ligands; cyclobutane; gelators; surfactants.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Dias R.S., Lindman B. In: DNA Interaction with Polymers and Surfactants. Dias R.S., Lindman B., editors. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2008.
-
- Escuder B., Miravet J.F. In: Functional Molecular Gels. Escuder B., Miravet J.F., editors. RSC Soft Matter Series Royal Society of Chemistry; Cambridge, UK: 2014.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

