Establishment of a Parvovirus B19 NS1-Expressing Recombinant Adenoviral Vector for Killing Megakaryocytic Leukemia Cells
- PMID: 31487941
- PMCID: PMC6783920
- DOI: 10.3390/v11090820
Establishment of a Parvovirus B19 NS1-Expressing Recombinant Adenoviral Vector for Killing Megakaryocytic Leukemia Cells
Abstract
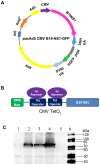
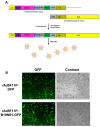
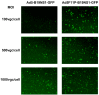
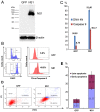
Adenoviral viral vectors have been widely used for gene-based therapeutics, but commonly used serotype 5 shows poor transduction efficiency into hematopoietic cells. In this study, we aimed to generate a recombinant adenovirus serotype 5 (rAd5) vector that has a high efficiency in gene transfer to megakaryocytic leukemic cells with anticancer potential. We first modified the rAd5 backbone vector with a chimeric fiber gene of Ad5 and Ad11p (rAd5F11p) to increase the gene delivery efficiency. Then, the nonstructural protein NS1 of human parvovirus B19 (B19V), which induces cell cycle arrest at the G2/M phase and apoptosis, was cloned into the adenoviral shuttle vector. As the expression of parvoviral NS1 protein inhibited Ad replication and production, we engineered the cytomegalovirus (CMV) promoter, which governs NS1 expression, with two tetracycline operator elements (TetO2). Transfection of the rAd5F11p proviral vectors in Tet repressor-expressing T-REx-293 cells produced rAd in a large quantity. We further evaluated this chimeric rAd5F11p vector in gene delivery in human leukemic cells, UT7/Epo-S1. Strikingly, the novel rAd5F11p-B19NS1-GFP vector, exhibited a transduction efficiency much higher than the original vector, rAd5-B19NS1-GFP, in UT7/Epo-S1 cells, in particular, when they were transduced at a relatively low multiplicity of infection (100 viral genome copies/cell). After the transduction of rAd5F11p-B19NS1-GFP, over 90% of the UT7/Epo-S1 cells were arrested at the G2/M phase, and approximately 40%-50% of the cells were undergoing apoptosis, suggesting the novel rAd5F11P-B19NS1-GFP vector holds a promise in therapeutic potentials of megakaryocytic leukemia.
Keywords: adenoviral vector; anti-cancer; apoptosis; cell cycle arrest; parvovirus B19.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Nilsson M., Ljungberg J., Richter J., Kiefer T., Magnusson M., Lieber A., Widegren B., Karlsson S., Fan X. Development of an adenoviral vector system with adenovirus serotype 35 tropism; efficient transient gene transfer into primary malignant hematopoietic cells. J. Gene Med. 2004;6:631–641. doi: 10.1002/jgm.543. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

