Carbohydrate, glutathione, and polyamine metabolism are central to Aspergillus flavus oxidative stress responses over time
- PMID: 31488075
- PMCID: PMC6727485
- DOI: 10.1186/s12866-019-1580-x
Carbohydrate, glutathione, and polyamine metabolism are central to Aspergillus flavus oxidative stress responses over time
Abstract
Background: The primary and secondary metabolites of fungi are critical for adaptation to environmental stresses, host pathogenicity, competition with other microbes, and reproductive fitness. Drought-derived reactive oxygen species (ROS) have been shown to stimulate aflatoxin production and regulate in Aspergillus flavus, and may function in signaling with host plants. Here, we have performed global, untargeted metabolomics to better understand the role of aflatoxin production in oxidative stress responses, and also explore isolate-specific oxidative stress responses over time.
Results: Two field isolates of A. flavus, AF13 and NRRL3357, possessing high and moderate aflatoxin production, respectively, were cultured in medium with and without supplementation with 15 mM H2O2, and mycelia were collected following 4 and 7 days in culture for global metabolomics. Overall, 389 compounds were described in the analysis which encompassed 9 biological super-pathways and 47 sub-pathways. These metabolites were examined for differential accumulation. Significant differences were observed in both isolates in response to oxidative stress and when comparing sampling time points.
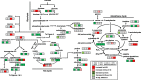
Conclusions: The moderately high aflatoxin-producing isolate, NRRL3357, showed extensive stimulation of antioxidant mechanisms and pathways including polyamines metabolism, glutathione metabolism, TCA cycle, and lipid metabolism while the highly aflatoxigenic isolate, AF13, showed a less vigorous response to stress. Carbohydrate pathway levels also imply that carbohydrate repression and starvation may influence metabolite accumulation at the later timepoint. Higher conidial oxidative stress tolerance and antioxidant capacity in AF13 compared to NRRL3357, inferred from their metabolomic profiles and growth curves over time, may be connected to aflatoxin production capability and aflatoxin-related antioxidant accumulation. The coincidence of several of the detected metabolites in H2O2-stressed A. flavus and drought-stressed hosts also suggests their potential role in the interaction between these organisms and their use as markers/targets to enhance host resistance through biomarker selection or genetic engineering.
Keywords: Aflatoxin; Aspergillus flavus; Drought stress; Metabolomics; Oxidative stress.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Achuo EA, Prinsen E, Höfte M. Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathol. 2006;55:178–186. doi: 10.1111/j.1365-3059.2006.01340.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

