Combined targeting of HER-2 and HER-3 represents a promising therapeutic strategy in colorectal cancer
- PMID: 31488078
- PMCID: PMC6727342
- DOI: 10.1186/s12885-019-6051-0
Combined targeting of HER-2 and HER-3 represents a promising therapeutic strategy in colorectal cancer
Abstract
Background: Abrogation of growth factor-dependent signaling represents an effective therapeutic strategy for patients with colorectal cancer (CRC). Here we evaluated the effectiveness of targeting the epidermal growth factor (EGF) receptors HER-2 and HER-3 in the three cell lines LS513, LS1034 and SW837.
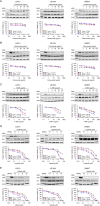
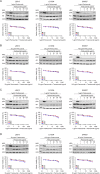
Methods: Treatment with HER-2-specific antibodies trastuzumab and pertuzumab resulted in a mild reduction of cellular viability. In contrast, the antibody-drug conjugate T-DM1 mediated a strong and dose-dependent decrease of viability and Akt phosphorylation.
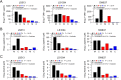
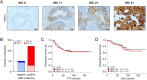
Results: The most striking effects were observed with the dual tyrosine kinase inhibitor lapatinib, and the Pan-ErbB inhibitor afatinib. Selectively, the effect of EGF receptor inhibition was augmented by a combination with 5-fluorouracil and oxaliplatin. Finally, high expression of HER-3 was detected in 121 of 172 locally advanced rectal cancers (70.3%). In conclusion, inhibition of EGF receptors effectively blocks downstream signaling and significantly impairs viability of CRC cells. However, the effectiveness of receptor inhibition highly depends on the inhibitors' mode of action, as targeting HER-2 alone is not sufficient.
Conclusion: Since HER-2 and HER-3 are expressed in a relevant number of patients, targeting both receptors may represent a promising therapeutic strategy for CRC.
Keywords: Colorectal cancer; HER-2; HER-3; Inhibitors; Targeted therapy.
Conflict of interest statement
The co-author T.B. is a member of the editorial board of BMC cancer. Beyond, the authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

