Ambulatory Toxicity Management (AToM) in patients receiving adjuvant or neo-adjuvant chemotherapy for early stage breast cancer - a pragmatic cluster randomized trial protocol
- PMID: 31488084
- PMCID: PMC6729066
- DOI: 10.1186/s12885-019-6099-x
Ambulatory Toxicity Management (AToM) in patients receiving adjuvant or neo-adjuvant chemotherapy for early stage breast cancer - a pragmatic cluster randomized trial protocol
Abstract
Background: Population-based studies suggest that emergency department visits and hospitalizations are common among patients receiving chemotherapy and that rates in routine practice are higher than expected from clinical trials. Chemotherapy-related toxicities are often predictable and, consequently, acute care visits may be preventable with adequate treatment planning and support between visits to the cancer centre. We will evaluate the impact of proactive telephone-based toxicity management on emergency department visits and hospitalizations in women with early stage breast cancer receiving chemotherapy.
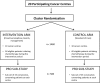
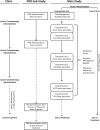
Methods: In this pragmatic covariate constraint-based cluster randomized trial, 20 centres in Ontario, Canada are randomly allocated to either proactive telephone toxicity management (intervention) or routine care (control). The primary outcome is the cluster-level mean number of ED + H visits per patient evaluated using Ontario administrative healthcare data. Participants are all patients with early stage (I-III) breast cancer commencing adjuvant or neo-adjuvant chemotherapy at participating institutions during the intervention period. At least 25 patients at each centre participate in a patient reported outcomes sub-study involving the collection of standardized questionnaires to measure: severity of treatment toxicities, self-care, self-efficacy, quality of life, and coordination of care. Patients participating in the patient reported outcomes (PRO) sub-study are asked to provide written consent to link their PRO data to administrative data. Unit costs will be applied to each per person resource utilized, and a total cost per population and patient will be generated. An incremental cost-effectiveness analysis will be undertaken to compare the incremental costs and outcomes between the intervention and control groups from the health system perspective.
Discussion: This study evaluates the effectiveness of a proactive toxicity management intervention in a routine care setting. The use of administrative healthcare data to evaluate the primary outcome enables an evaluation in a real world setting and at a much larger scale than previous studies.
Trial registration: Clinicaltrials.gov , NCT02485678. Registered 30 June 2015.
Keywords: Breast cancer; Chemotherapy toxicity; Quality improvement; Symptom management; Telephone case management.
Conflict of interest statement
Authors CCE and EG hold appointments at the Ontario Institute for Cancer Research (OICR) Health Services Research Program. All other authors have no competing interests to declare.
Figures
References
-
- Cancer Quality Council of Ontario. Cancer System Quality Index (CSQI). 2017. http://www.csqi.on.ca/. Accessed on 23 Feb 2018.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

