Intracellular human antibody fragments recognizing the VP35 protein of Zaire Ebola filovirus inhibit the protein activity
- PMID: 31488108
- PMCID: PMC6727353
- DOI: 10.1186/s12896-019-0554-2
Intracellular human antibody fragments recognizing the VP35 protein of Zaire Ebola filovirus inhibit the protein activity
Abstract
Background: Ebola hemorrhagic fever is caused by the Ebola filovirus (EBOV), which is one of the most aggressive infectious agents known worldwide. The EBOV pathogenesis starts with uncontrolled viral replication and subversion of both the innate and adaptive host immune response. The multifunctional viral VP35 protein is involved in this process by exerting an antagonistic action against the early antiviral alpha/beta interferon (IFN-α/β) response, and represents a suitable target for the development of strategies to control EBOV infection. Phage display technology permits to select antibodies as single chain Fragment variable (scFv) from an artificial immune system, due to their ability to specifically recognize the antigen of interest. ScFv is ideal for genetic manipulation and to obtain antibody constructs useful for targeting either antigens expressed on cell surface or intracellular antigens if the scFv is expressed as intracellular antibody (intrabody) or delivered into the cells.
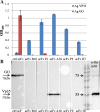
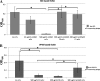
Results: Monoclonal antibodies (mAb) in scFv format specific for the EBOV VP35 were isolated from the ETH-2 library of human recombinant antibodies by phage display technology. Five different clones were identified by sequencing, produced in E.coli and expressed in CHO mammalian cells to be characterized in vitro. All the selected scFvs were able to react with recombinant VP35 protein in ELISA, one of the scFvs being also able to react in Western Blot assay (WB). In addition, all scFvs were expressed in cell cytoplasm as intrabodies; a luciferase reporter gene inhibition assay performed in A549 cells showed that two of the scFvs can significantly hamper the inhibition of the IFN-β-induced RIG-I signaling cascade mediated by EBOV VP35.
Conclusion: Five antibodies in scFv format recognize an active form of EBOV VP35 in ELISA, while one antibody also recognizes VP35 in WB. Two of these scFvs were also able to interfere with the intracellular activity of VP35 in a cell system in vitro. These findings suggest that such antibodies in scFv format might be employed to develop therapeutic molecules able to hamper EBOV infections.
Keywords: Intrabody; VP35; Zaire ebolavirus; scFv.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Goldstein T, Anthony SJ, Gbakima A, Bird BH, Bangura J, Tremeau-Bravard A, Belaganahalli MN, Wells HL, Dhanota JK, Liang E, Grodus M, Jangra RH, Dejesus VA, Lasso G, Smith BR, Jambai A, Kamara BO, Kamara S, Bangura W, Monagin C, Shapira S, Johnson CK, Saylors K, Rubin EM, Chandran K, Lipkin WI, Mazet J. The discovery of a new Ebolavirus, Bombali virus, adds further support for bats as hosts of Ebolaviruses. Int J Infect Dis. 2019;79(Supp 1):4–5. doi: 10.1016/j.ijid.2018.11.030. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

