Immune checkpoint inhibitors of PD-L1 as cancer therapeutics
- PMID: 31488176
- PMCID: PMC6729004
- DOI: 10.1186/s13045-019-0779-5
Immune checkpoint inhibitors of PD-L1 as cancer therapeutics
Abstract
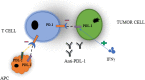
Since the discovery of immune checkpoint proteins, there has been a special interest in developing antibodies that block programmed cell death 1 receptor (PD-1) and programmed cell death receptor ligand 1 (PD-L1) for a subset of cancer patients. PD-1 signaling negatively regulates T cell-mediated immune responses and serves as a mechanism for tumors to evade an antigen-specific T cell immunologic response. It plays a role in promoting cancer development and progression by enhancing tumor cell survival. With this background, PD-1 signaling represents a valuable therapeutic target for novel and effective cancer immunotherapy. Clinical data shows that blockade of this PD-1 signaling significantly enhance antitumor immunity, produce durable clinical responses, and prolong survival. Currently, there are three FDA-approved PD-L1 inhibitors for various malignancies ranging from non-small cell lung cancer to Merkel cell carcinoma. This review is to summarize many ongoing phase II/III trials of atezolizumab, durvalumab, avelumab, and new PD-L1 inhibitors in clinical developments. In particular, we focus on key trials that paved the pathway to FDA-approved indications for atezolizumab, durvalumab, and avelumab. Despite the popularity and accelerated FDA approval of PD-L1 inhibitors, further considerations into predictive biomarkers, mechanisms of resistance, treatment duration, immune-related toxicities, and PD-L1 expression threshold are needed to optimize anticancer potential in this class of immunotherapy.
Keywords: Companion diagnostics assays; Immune checkpoints; Merkel cell carcinoma; Non-small cell lung cancer; T cell dysfunction; Tumor-infiltrating lymphocytes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

