Regulation of Drosophila Intestinal Stem Cell Proliferation by Enterocyte Mitochondrial Pyruvate Metabolism
- PMID: 31488514
- PMCID: PMC6829144
- DOI: 10.1534/g3.119.400633
Regulation of Drosophila Intestinal Stem Cell Proliferation by Enterocyte Mitochondrial Pyruvate Metabolism
Abstract
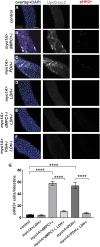
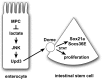
Multiple signaling pathways in the adult Drosophila enterocyte sense cellular damage or stress and signal to intestinal stem cells (ISCs) to undergo proliferation and differentiation, thereby maintaining intestinal homeostasis. Here we show that misregulation of mitochondrial pyruvate metabolism in enterocytes can stimulate ISC proliferation and differentiation. Our studies focus on the Mitochondrial Pyruvate Carrier (MPC), which is an evolutionarily-conserved protein complex that resides in the inner mitochondrial membrane and transports cytoplasmic pyruvate into the mitochondrial matrix. Loss of MPC function in enterocytes induces Unpaired cytokine expression, which activates the JAK/STAT pathway in ISCs, promoting their proliferation. Upd3 and JNK signaling are required in enterocytes for ISC proliferation, indicating that this reflects a canonical non-cell autonomous damage response. Disruption of lactate dehydrogenase in enterocytes has no effect on ISC proliferation but it suppresses the proliferative response to a loss of enterocyte MPC function, suggesting that lactate contributes to this pathway. These studies define an important role for cellular pyruvate metabolism in differentiated enterocytes to maintain stem cell proliferation rates.
Keywords: intestinal homeostasis; metabolism; mitochondria; stem cells.
Copyright © 2019 Wisidagama, Thummel.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
