Interaction of the tetratricopeptide repeat domain of aryl hydrocarbon receptor-interacting protein-like 1 with the regulatory Pγ subunit of phosphodiesterase 6
- PMID: 31488544
- PMCID: PMC6816093
- DOI: 10.1074/jbc.RA119.010666
Interaction of the tetratricopeptide repeat domain of aryl hydrocarbon receptor-interacting protein-like 1 with the regulatory Pγ subunit of phosphodiesterase 6
Abstract
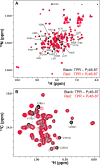
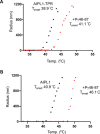
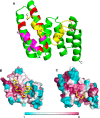
Phosphodiesterase-6 (PDE6) is key to both phototransduction and health of rods and cones. Proper folding of PDE6 relies on the chaperone activity of aryl hydrocarbon receptor-interacting protein-like 1 (AIPL1), and mutations in both PDE6 and AIPL1 can cause a severe form of blindness. Although AIPL1 and PDE6 are known to interact via the FK506-binding protein domain of AIPL1, the contribution of the tetratricopeptide repeat (TPR) domain of AIPL1 to its chaperone function is poorly understood. Here, we demonstrate that AIPL1-TPR interacts specifically with the regulatory Pγ subunit of PDE6. Use of NMR chemical shift perturbation (CSP) mapping technique revealed the interface between the C-terminal portion of Pγ and AIPL1-TPR. Our solution of the crystal structure of the AIPL1-TPR domain provided additional information, which together with the CSP data enabled us to generate a model of this interface. Biochemical analysis of chimeric AIPL1-AIP proteins supported this model and also revealed a correlation between the affinity of AIPL1-TPR for Pγ and the ability of Pγ to potentiate the chaperone activity of AIPL1. Based on these results, we present a model of the larger AIPL1-PDE6 complex. This supports the importance of simultaneous interactions of AIPL1-FK506-binding protein with the prenyl moieties of PDE6 and AIPL1-TPR with the Pγ subunit during the folding and/or assembly of PDE6. This study sheds new light on the versatility of TPR domains in protein folding by describing a novel TPR-protein binding partner, Pγ, and revealing that this subunit imparts AIPL1 selectivity for its client.
Keywords: HSP90; X-ray crystallography; chaperone; nuclear magnetic resonance (NMR); phosphodiesterases; photoreceptor; phototransduction; small-angle X-ray scattering (SAXS); tetratricopeptide repeat domain.
© 2019 Yadav et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

