Synergistic effects of PRIMA-1Met (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia
- PMID: 31488557
- PMCID: PMC7271596
- DOI: 10.3324/haematol.2019.218453
Synergistic effects of PRIMA-1Met (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia
Abstract
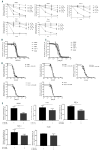
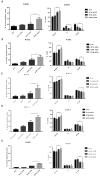
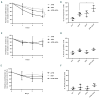
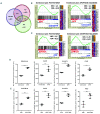
Myelodysplastic syndromes and acute myeloid leukemia with TP53 mutations are characterized by frequent relapses, poor or short responses, and poor survival with the currently available therapies including chemotherapy and 5-azacitidine (AZA). PRIMA-1Met(APR-246,APR) is a methylated derivative of PRIMA-1, which induces apoptosis in human tumor cells through restoration of the transcriptional transactivation function of mutant p53. Here we show that low doses of APR on its own or in combination with AZA reactivate the p53 pathway and induce an apoptosis program. Functionally, we demonstrate that APR exerts these activities on its own and that it synergizes with AZA in TP53-mutated myelodysplastic syndromes (MDS)/acute myeloid leukemia (AML) cell lines and in TP53-mutated primary cells from MDS/AML patients. Low doses of APR on its own or in combination with AZA also show significant efficacy in vivo Lastly, using transcriptomic analysis, we found that the APR + AZA synergy was mediated by downregulation of the FLT3 pathway in drug-treated cells. Activation of the FLT3 pathway by FLT3 ligand reversed the inhibition of cell proliferation by APR + AZA. These data suggest that TP53-mutated MDS/AML may be better targeted by the addition of APR-246 to conventional treatments.
Copyright© 2020 Ferrata Storti Foundation.
Figures



Comment in
-
To target the untargetable: elucidation of synergy of APR-246 and azacitidine in TP53 mutant myelodysplastic syndromes and acute myeloid leukemia.Haematologica. 2020 Jun;105(6):1470-1472. doi: 10.3324/haematol.2020.249060. Haematologica. 2020. PMID: 32482751 Free PMC article. No abstract available.
References
-
- Adès L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet. 2014; 383(9936):2239–2252. - PubMed
-
- Haferlach C, Dicker F, Herholz H, Schnittger S, Kern W, Haferlach T. Mutations of the TP53 gene in acute myeloid leukemia are strongly associated with a complex aberrant karyotype. Leukemia 2008; 22(8):1539–1541. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

