Porcine deltacoronavirus causes diarrhea in various ages of field-infected pigs in China
- PMID: 31488617
- PMCID: PMC6746998
- DOI: 10.1042/BSR20190676
Porcine deltacoronavirus causes diarrhea in various ages of field-infected pigs in China
Abstract
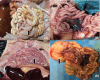
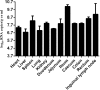
Porcine deltacoronavirus (PDCoV) is a novel coronavirus that causes acute diarrhea in suckling piglets. In Henan province of China, three swine farms broke out diarrhea in different ages of pigs during June of 2017, March of 2018 and January of 2019, respectively. PCR method, Taqman real-time RT-PCR method, sequencing, histopathology and immunohistochemistry (IHC) were conducted with the collected samples, and the results showed that PDCoV was detected among the suckling piglets, commercial fattening pigs and sows with diarrhea. PDCoV-infected suckling piglets were characterized with thin and transparent intestinal walls from colon to caecum, spot hemorrhage at mesentery and intestinal bleeding. PDCoV RNA was detected in multiple organs and tissues by Taqman real-time RT-PCR, which had high copies in ileum, inguinal lymph node, rectum and spleen. PDCoV antigen was detected in the basal layer of jejunum and ileum by IHC. In this research, we found that PDCoV could infect various ages of farmed pigs with watery diarrhea and anorexia in different seasons in a year.
Keywords: Diarrhea; Histopathology; PDCoV; Pig age; qRT-PCR.
© 2019 The Author(s).
Conflict of interest statement
Ethics Statement
The research protocol for animal experiments of live pigs in the present study was approved by the Animal Care and Use Committee of Henan Agricultural University (Zhengzhou, China) and was performed in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, China).
Figures


References
-
- Woo P.C., Lau S.K., Lam C.S.. et al. (2012) Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 86, 3995–4008 10.1128/JVI.06540-11 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

