Neural sensitization improves encoding fidelity in the primate retina
- PMID: 31488831
- PMCID: PMC6728337
- DOI: 10.1038/s41467-019-11734-4
Neural sensitization improves encoding fidelity in the primate retina
Abstract
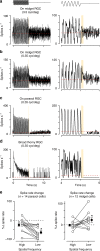
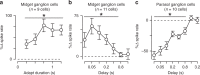
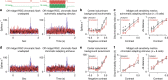
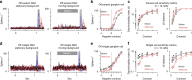
An animal's motion through the environment can induce large and frequent fluctuations in light intensity on the retina. These fluctuations pose a major challenge to neural circuits tasked with encoding visual information, as they can cause cells to adapt and lose sensitivity. Here, we report that sensitization, a short-term plasticity mechanism, solves this difficult computational problem by maintaining neuronal sensitivity in the face of these fluctuations. The numerically dominant output pathway in the macaque monkey retina, the midget (parvocellular-projecting) pathway, undergoes sensitization under specific conditions, including simulated eye movements. Sensitization is present in the excitatory synaptic inputs from midget bipolar cells and is mediated by presynaptic disinhibition from a wide-field mechanism extending >0.5 mm along the retinal surface. Direct physiological recordings and a computational model indicate that sensitization in the midget pathway supports accurate sensory encoding and prevents a loss of responsiveness during dynamic visual processing.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Barlow, H. B. in Current Problems in Animal Behaviour (eds Thorpe, W. H. & Zangwill, O. L.) 331–360 (Cambridge University Press, Cambridge, 1961).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

