Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress
- PMID: 31488841
- PMCID: PMC6728379
- DOI: 10.1038/s41467-019-12045-4
Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress
Abstract
Timely perception of adverse environmental changes is critical for survival. Dynamic changes in gases are important cues for plants to sense environmental perturbations, such as submergence. In Arabidopsis thaliana, changes in oxygen and nitric oxide (NO) control the stability of ERFVII transcription factors. ERFVII proteolysis is regulated by the N-degron pathway and mediates adaptation to flooding-induced hypoxia. However, how plants detect and transduce early submergence signals remains elusive. Here we show that plants can rapidly detect submergence through passive ethylene entrapment and use this signal to pre-adapt to impending hypoxia. Ethylene can enhance ERFVII stability prior to hypoxia by increasing the NO-scavenger PHYTOGLOBIN1. This ethylene-mediated NO depletion and consequent ERFVII accumulation pre-adapts plants to survive subsequent hypoxia. Our results reveal the biological link between three gaseous signals for the regulation of flooding survival and identifies key regulatory targets for early stress perception that could be pivotal for developing flood-tolerant crops.
Conflict of interest statement
The authors declare no competing interests.
Figures

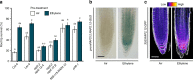
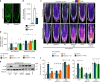
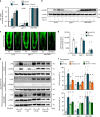

Comment in
-
Ethylene Signaling Controls Fast Oxygen Sensing in Plants.Trends Plant Sci. 2020 Jan;25(1):3-6. doi: 10.1016/j.tplants.2019.10.010. Epub 2019 Nov 13. Trends Plant Sci. 2020. PMID: 31734094
References
-
- Hirabayashi Y, et al. Global flood risk underclimate change. Nat. Clim. Chang. 2013;3:816–821. doi: 10.1038/nclimate1911. - DOI
-
- Shiono K, Takahashi H, Colmer TD, Nakazono M. Role of ethylene in acclimations to promote oxygen transport in roots of plants in waterlogged soils. Plant Sci. 2008;175:52–58. doi: 10.1016/j.plantsci.2008.03.002. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

