CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR)
- PMID: 31489471
- PMCID: PMC7160220
- DOI: 10.1007/s00330-019-06357-8
CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR)
Erratum in
-
Correction to: CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR).Eur Radiol. 2020 Jul;30(7):4143-4144. doi: 10.1007/s00330-020-06741-9. Eur Radiol. 2020. PMID: 32124024 Free PMC article.
Abstract
Transcatheter aortic valve replacement (TAVR) is a minimally invasive alternative to conventional aortic valve replacement in symptomatic patients with severe aortic stenosis and contraindications to surgery. The procedure has shown to improve patient's quality of life and prolong short- and mid-term survival in high-risk individuals, becoming a widely accepted therapeutic option which has been integrated into current clinical guidelines for the management of valvular heart disease. Nevertheless, not every patient at high-risk for surgery is a good candidate for TAVR. Besides clinical selection, which is usually established by the Heart Team, certain technical and anatomic criteria must be met as, unlike in surgical valve replacement, annular sizing is not performed under direct surgical evaluation but on the basis of non-invasive imaging findings. Present consensus document was outlined by a working group of researchers from the European Society of Cardiovascular Radiology (ESCR) and aims to provide guidance on the utilisation of CT and MR imaging prior to TAVR. Particular relevance is given to the technical requirements and standardisation of the scanning protocols which have to be tailored to the remarkable variability of the scanners currently utilised in clinical practice; recommendations regarding all required pre-procedural measurements and medical reporting standardisation have been also outlined, in order to ensure quality and consistency of reported data and terminology. KEY POINTS: • To provide a reference document for CT and MR acquisition techniques, taking into account the significant technological variation of available scanners. • To review all relevant measurements that are required and define a step-by-step guided approach for the measurements of different structures implicated in the procedure. • To propose a CT/MR reporting template to assist in consistent communication between various sites and specialists involved in the procedural planning.
Keywords: Aortic valve stenosis; Consensus; Magnetic resonance imaging; Multidetector computed tomography; Transcatheter aortic valve replacement.
Conflict of interest statement
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Figures










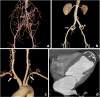
References
-
- Bach DS, Siao D, Girard SE, Duvernoy C, McCallister BD, Jr, Gualano SK. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes. 2009;2:533–539. - PubMed
-
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–2720. - PubMed
-
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008. - PubMed
-
- Hamm CW, Arsalan M, Mack MJ. The future of transcatheter aortic valve implantation. Eur Heart J. 2016;37:803–810. - PubMed
-
- Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

