Exosomes from miR-20b-3p-overexpressing stromal cells ameliorate calcium oxalate deposition in rat kidney
- PMID: 31489770
- PMCID: PMC6815912
- DOI: 10.1111/jcmm.14555
Exosomes from miR-20b-3p-overexpressing stromal cells ameliorate calcium oxalate deposition in rat kidney
Abstract
Hyperoxaluria-induced calcium oxalate (CaOx) deposition is the key factor in kidney stone formation, for which adipose-derived stromal cells (ADSCs) have been used as a therapeutic treatment. Studies revealed that miR-20b-3p is down-regulated in hypercalciuric stone-forming rat kidney. To investigate whether ADSC-derived miR-20b-3p-enriched exosomes protect against kidney stones, an ethylene glycol (EG)-induced hyperoxaluria rat model and an in vitro model of oxalate-induced NRK-52E cells were established to explore the protective mechanism of miR-20b-3p. The results showed that miR-20b-3p levels were decreased following hyperoxaluria in the urine of patients and in kidney tissues from animal models. Furthermore, treatment with miR-20b-3p-enriched exosomes from ADSCs protected EG-induced hyperoxaluria rats, and cell experiments confirmed that co-culture with miR-20b-3p-enriched exosomes alleviated oxalate-induced cell autophagy and the inflammatory response by inhibiting ATG7 and TLR4. In conclusion, ADSC-derived miR-20b-3p-enriched exosomes protected against kidney stones by suppressing autophagy and inflammatory responses.
Keywords: calcium oxalate deposition; exosomes; miR-20b-3p; microRNAs; stromal cells.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures
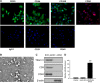

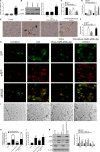


Comment in
-
Re: Exosomes from miR-20b-3p-Overexpressing Stromal Cells Ameliorate Calcium Oxalate Deposition in Rat Kidney.J Urol. 2020 Feb;203(2):246. doi: 10.1097/JU.0000000000000633. Epub 2019 Nov 5. J Urol. 2020. PMID: 31689150 No abstract available.
References
-
- Zeng G, Mai Z, Xia S, et al. Prevalence of kidney stones in China: an ultrasonography based cross‐sectional study. BJU Int. 2017;120:109‐116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

