Serrulin: A Glycine-Rich Bioactive Peptide from the Hemolymph of the Yellow Tityus serrulatus Scorpion
- PMID: 31489876
- PMCID: PMC6784228
- DOI: 10.3390/toxins11090517
Serrulin: A Glycine-Rich Bioactive Peptide from the Hemolymph of the Yellow Tityus serrulatus Scorpion
Abstract
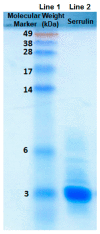
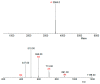
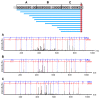
Antimicrobial peptides (AMPs) are small molecules, which have a potential use as antibiotic or pharmacological tools. In chelicerate organisms, such as scorpions, these molecules constitute an alternative defense system against microorganisms. The aim of this work was to identify AMPs in the hemolymph of the Tityus serrulatus scorpion. Fractions of plasma and hemocytes were subjected to high-performance liquid chromatography (HPLC) and then analyzed to determine their activity in inhibiting microbial growth. One of the fractions from the hemocytes presents antimicrobial activity against microorganisms, such as Gram-negative and Gram-positive bacteria, fungi, and yeast. These fractions were analyzed by mass spectrometry, and a fragment of 3564 Da. was identified. The peptide was called serrulin, because it is derived from the species T. serrulatus. A comparison of the amino acid sequence of serrulin with databases shows that it has a similarity to the glycine-rich peptides described in Cupienius salai and Acanthoscurria gomesiana (spiders). Furthermore, serrulin has no hemolytic activity against human erythrocytes. While the presence of AMPs in T. serrulatus venom has been described in other works, this is the first work to characterize the presence of these molecules in the hemolymph (hemocytes) of this species and show its potential use as an alternative to conventional antibiotics against different species of microorganisms.
Keywords: Tityus serrulatus; antimicrobial peptide; glycine-rich peptide; innate immune system; scorpions.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Guo X., Ma C., Du Q., Wei R., Wang L., Zhou M., Chen T., Shaw C. Two peptides, TsAP-1 and TsAP-2, from the venom of the Brazilian yellow scorpion, Tityus serrulatus: Evaluation of their antimicrobial and anticancer activities. Biochimie. 2013;95:1784–1794. doi: 10.1016/j.biochi.2013.06.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

