Gentamicin Targets Acid Sphingomyelinase in Cancer: The Case of the Human Gastric Cancer NCI-N87 Cells
- PMID: 31489901
- PMCID: PMC6770866
- DOI: 10.3390/ijms20184375
Gentamicin Targets Acid Sphingomyelinase in Cancer: The Case of the Human Gastric Cancer NCI-N87 Cells
Abstract
Emerging literature implicates acid sphingomyelinase in tumor sensitivity/resistance to anticancer treatments. Gentamicin is a drug commonly used as an antimicrobial but its serendipity effects have been shown. Even though many evidences on the role of gentamicin in cancer have been reported, its mechanism of action is poorly understood. Here, we explored acid sphingomyelinase as a possible new target of gentamicin in cancer. Since gastric cancer is one of the most common cancers and represents the second cause of death in the world, we performed the study in NCI-N87 gastric cancer cell line. The effect of the drug resulted in the inhibition of cell proliferation, including a reduction of cell number and viability, in the decrease of MIB-1 proliferative index as well as in the upregulation of cyclin-dependent kinase inhibitor 1A and 1B (CDKN1A and CDKN1B), and growth arrest and DNA-damage 45A (GADD45A) genes. The cytotoxicity was apoptotic as shown by FACS analysis. Additionally, gentamicin reduced HER2 protein, indicating a minor tumor aggressiveness. To further define the involvement of sphingomyelin metabolism in the response to the drug, gene and protein expression of acid and neutral sphingomeylinase was analyzed in comparison with phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and vitamin D receptor (VDR), molecules involved in cancer. Gentamicin induced a downregulation of PTEN, VDR, and neutral sphingomyelinase and a strong upregulation of acid sphingomyelinase. Of note, we identified the same upregulation of acid sphingomyelinase upon gentamicin treatment in other cancer cells and not in normal cells. These findings provide new insights into acid sphingomyelinase as therapeutic target, reinforcing studies on the potential role of gentamicin in anticancer therapy.
Keywords: cancer; drug target; lipid; sphingomyelin; sphingomyelinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

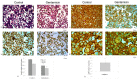

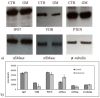

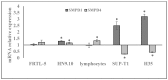

References
-
- Albi E. Role of intranuclear lipids in health and disease. Clin. Lipidol. 2011;6:59–69. doi: 10.2217/clp.10.83. - DOI
-
- Ito H., Murakami M., Furuhata A., Gao S., Yoshida K., Sobue S., Hagiwara K., Takagi A., Kojima T., Suzuki M., et al. Transcriptional regulation of neutral sphingomyelinase 2 gene expression of a human breast cancer cell line, MCF-7, induced by the anti-cancer drug, daunorubicin. Biochim. Biophys. Acta. 2009;1789:681–690. doi: 10.1016/j.bbagrm.2009.08.006. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

