A Review on Coordination Properties of Thiol-Containing Chelating Agents Towards Mercury, Cadmium, and Lead
- PMID: 31489907
- PMCID: PMC6767255
- DOI: 10.3390/molecules24183247
A Review on Coordination Properties of Thiol-Containing Chelating Agents Towards Mercury, Cadmium, and Lead
Abstract
The present article reviews the clinical use of thiol-based metal chelators in intoxications and overexposure with mercury (Hg), cadmium (Cd), and lead (Pb). Currently, very few commercially available pharmaceuticals can successfully reduce or prevent the toxicity of these metals. The metal chelator meso-2,3-dimercaptosuccinic acid (DMSA) is considerably less toxic than the classical agent British anti-Lewisite (BAL, 2,3-dimercaptopropanol) and is the recommended agent in poisonings with Pb and organic Hg. Its toxicity is also lower than that of DMPS (dimercaptopropane sulfonate), although DMPS is the recommended agent in acute poisonings with Hg salts. It is suggested that intracellular Cd deposits and cerebral deposits of inorganic Hg, to some extent, can be mobilized by a combination of antidotes, but clinical experience with such combinations are lacking. Alpha-lipoic acid (α-LA) has been suggested for toxic metal detoxification but is not considered a drug of choice in clinical practice. The molecular mechanisms and chemical equilibria of complex formation of the chelators with the metal ions Hg2+, Cd2+, and Pb2+ are reviewed since insight into these reactions can provide a basis for further development of therapeutics.
Keywords: BAL; DMPS; DMSA; metal chelator; metal ion.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
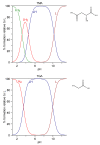







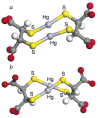

References
-
- ATSDR . ATSDR Substance Priority List. ATSDR; Atlanta, GA, USA: 2017.
-
- World Health Organisation . Ten Chemicals of Major Public Health Concern. WHO; Geneva, Switzerland: 2010. pp. 1–4.
-
- Pearson R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963;85:3533–3539. doi: 10.1021/ja00905a001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

