No Evidence for a Role for Antibodies during Vaccination-Induced Enhancement of Porcine Reproductive and Respiratory Syndrome
- PMID: 31489915
- PMCID: PMC6784192
- DOI: 10.3390/v11090829
No Evidence for a Role for Antibodies during Vaccination-Induced Enhancement of Porcine Reproductive and Respiratory Syndrome
Abstract
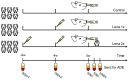
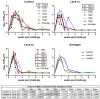
Vaccination is one of the most important tools to protect pigs against infection with porcine reproductive and respiratory syndrome virus 1 (PRRSV-1). Although neutralizing antibodies are considered to represent an important mechanism of protective immunity, anti-PRRSV antibodies, in particular at subneutralizing concentrations, have also been reported to exacerbate PRRSV infection, probably through FcγR-mediated uptake of antibody-opsonized PRRSV, resulting in enhanced infection of, and replication in, target cells. Therefore, we investigated this pathway using sera from an animal experiment in which vaccine-mediated enhancement of clinical symptoms was observed. Three groups of six pigs were vaccinated with an inactivated PRRSV vaccine based on the PRRSV-1 subtype 3 strain Lena and challenged after a single or a prime-boost immunization protocol, or injected with PBS. We specifically tested if sera obtained from these animals can enhance macrophage infections, viral shedding, or cytokine release at different dilutions. Neither the presence of neutralizing antibodies nor general anti-PRRSV antibodies, mediated an enhanced infection, increased viral release or cytokine production by macrophages. Taken together, our data indicate that the exacerbated disease was not caused by antibodies.
Keywords: PRRSV-1; antibody-dependent enhancement (ADE) of disease; homologous challenge; in vivo; inactivated vaccine; monocyte-derived macrophages.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
A porcine reproductive and respiratory syndrome virus candidate vaccine based on the synthetic attenuated virus engineering approach is attenuated and effective in protecting against homologous virus challenge.Vaccine. 2016 Nov 4;34(46):5546-5553. doi: 10.1016/j.vaccine.2016.09.049. Epub 2016 Oct 11. Vaccine. 2016. PMID: 27742217
-
Antibody response and maternal immunity upon boosting PRRSV-immune sows with experimental farm-specific and commercial PRRSV vaccines.Vet Microbiol. 2013 Dec 27;167(3-4):260-71. doi: 10.1016/j.vetmic.2013.08.017. Epub 2013 Aug 28. Vet Microbiol. 2013. PMID: 24041768
-
Lymphocyte activation as cytokine gene expression and secretion is related to the porcine reproductive and respiratory syndrome virus (PRRSV) isolate after in vitro homologous and heterologous recall of peripheral blood mononuclear cells (PBMC) from pigs vaccinated and exposed to natural infection.Vet Immunol Immunopathol. 2013 Feb 15;151(3-4):193-206. doi: 10.1016/j.vetimm.2012.11.006. Epub 2012 Nov 19. Vet Immunol Immunopathol. 2013. PMID: 23228653
-
Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus.Vet Immunol Immunopathol. 2015 Sep 15;167(1-2):1-14. doi: 10.1016/j.vetimm.2015.07.003. Epub 2015 Jul 17. Vet Immunol Immunopathol. 2015. PMID: 26209116 Free PMC article. Review.
-
Immunity to PRRSV: double-edged sword.Vet Microbiol. 1997 Apr;55(1-4):265-76. doi: 10.1016/s0378-1135(96)01327-2. Vet Microbiol. 1997. PMID: 9220622 Free PMC article. Review.
Cited by
-
Progress in PRRSV Infection and Adaptive Immune Response Mechanisms.Viruses. 2023 Jun 27;15(7):1442. doi: 10.3390/v15071442. Viruses. 2023. PMID: 37515130 Free PMC article. Review.
-
Porcine Reproductive and Respiratory Syndrome Virus: Immune Escape and Application of Reverse Genetics in Attenuated Live Vaccine Development.Vaccines (Basel). 2021 May 10;9(5):480. doi: 10.3390/vaccines9050480. Vaccines (Basel). 2021. PMID: 34068505 Free PMC article. Review.
-
Antibody dependent enhancement: Unavoidable problems in vaccine development.Adv Immunol. 2021;151:99-133. doi: 10.1016/bs.ai.2021.08.003. Epub 2021 Sep 14. Adv Immunol. 2021. PMID: 34656289 Free PMC article.
-
Efficacy of a live attenuated highly pathogenic PRRSV vaccine against a NADC30-like strain challenge: implications for ADE of PRRSV.BMC Vet Res. 2021 Jul 31;17(1):260. doi: 10.1186/s12917-021-02957-z. BMC Vet Res. 2021. PMID: 34332554 Free PMC article.
-
Fitness adaptations of Japanese encephalitis virus in pigs following vector-free serial passaging.PLoS Pathog. 2024 Aug 26;20(8):e1012059. doi: 10.1371/journal.ppat.1012059. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39186783 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

