Protoisomerization of Indigo and Isoindigo Dyes Confirmed by Gas-Phase Infrared Ion Spectroscopy
- PMID: 31490692
- PMCID: PMC6767361
- DOI: 10.1021/acs.jpca.9b06858
Protoisomerization of Indigo and Isoindigo Dyes Confirmed by Gas-Phase Infrared Ion Spectroscopy
Abstract
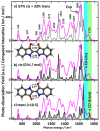
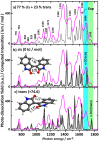
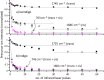
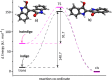
Gas-phase infrared multiple-photon dissociation (IRMPD) spectra are recorded for the protonated dye molecules indigo and isoindigo by using a quadrupole ion trap (QIT) mass spectrometer coupled to the free electron laser for infrared experiments (FELIX). From their fingerprint IR spectra (600-1800 cm-1) and comparison with quantum-chemical calculations at the density functional level of theory (B3LYP/6-31++G(d,p)), we derive their structures. We focus particularly on the question of whether trans-to-cis isomerization occurs upon protonation and transfer to the gas phase. The trans-configuration is energetically favored in the neutral forms of the dyes in solution and in the gas phase. Instead, the cis-isomer is lower in energy for the protonated forms of both species, but indigo is also notorious for not undergoing double-bond trans-to-cis isomerization, in contrast to many other conjugated systems. The IR spectra suggest that protoisomerization from trans to cis indeed occurs for both dyes. To estimate the extent of isomerization, on-resonance kinetics are measured on diagnostic and common vibrational frequencies to determine the ratio of cis-to-trans isomers. We find ratios of 65-70% cis and 30-35% trans for indigo versus 75-80% cis and 20-25% trans for isoindigo. Transition-state calculations for the isomerization reactions have been carried out, which indeed suggest a lower barrier for protonated isoindigo, qualitatively explaining the more efficient isomerization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Photochromic derivatives of indigo: historical overview of development, challenges and applications.Beilstein J Org Chem. 2024 Feb 7;20:228-242. doi: 10.3762/bjoc.20.23. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38352070 Free PMC article. Review.
-
IRMPD Spectra of Protonated Hydroxybenzaldehydes: Evidence of Torsional Barriers in Carboxonium Ions.Chemphyschem. 2020 Apr 20;21(8):749-761. doi: 10.1002/cphc.202000041. Epub 2020 Mar 11. Chemphyschem. 2020. PMID: 31951044
-
Molecular structure of the trans and cis isomers of metal-free phthalocyanine studied by gas-phase electron diffraction and high-level quantum chemical calculations: NH tautomerization and calculated vibrational frequencies.J Phys Chem A. 2008 May 29;112(21):4853-60. doi: 10.1021/jp801284c. Epub 2008 May 6. J Phys Chem A. 2008. PMID: 18457372
-
Modified Quadrupole Ion Trap Mass Spectrometer for Infrared Ion Spectroscopy: Application to Protonated Thiated Uridines.J Am Soc Mass Spectrom. 2018 Nov;29(11):2125-2137. doi: 10.1007/s13361-018-2047-2. Epub 2018 Aug 22. J Am Soc Mass Spectrom. 2018. PMID: 30136214
-
TOLUENIUM AND OTHER GASEOUS METHYLBENZENIUM IONS: COMPLEX INTERPLAY OF PROTONATED ARENES AND CYCLO-OLEFINS.Mass Spectrom Rev. 2021 Nov;40(6):741-781. doi: 10.1002/mas.21631. Epub 2020 May 29. Mass Spectrom Rev. 2021. PMID: 32468717 Review.
Cited by
-
Infrared Ion Spectroscopic Characterization of the Gaseous [Co(15-crown-5)(H2O)]2+ Complex.J Phys Chem A. 2023 Aug 31;127(34):7256-7263. doi: 10.1021/acs.jpca.3c04241. Epub 2023 Aug 18. J Phys Chem A. 2023. PMID: 37595154 Free PMC article.
-
Photochromic derivatives of indigo: historical overview of development, challenges and applications.Beilstein J Org Chem. 2024 Feb 7;20:228-242. doi: 10.3762/bjoc.20.23. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38352070 Free PMC article. Review.
-
Disassembly of Amyloid Fibril with Infrared Free Electron Laser.Int J Mol Sci. 2023 Feb 12;24(4):3686. doi: 10.3390/ijms24043686. Int J Mol Sci. 2023. PMID: 36835098 Free PMC article. Review.
-
Predicting the substituent effects in the optical and electrochemical properties of N,N'-substituted isoindigos.Photochem Photobiol Sci. 2021 Jul;20(7):927-938. doi: 10.1007/s43630-021-00071-5. Epub 2021 Jul 5. Photochem Photobiol Sci. 2021. PMID: 34227039 Free PMC article.
-
Application study of infrared free-electron lasers towards the development of amyloidosis therapy.J Synchrotron Radiat. 2022 Sep 1;29(Pt 5):1133-1140. doi: 10.1107/S1600577522007330. Epub 2022 Aug 12. J Synchrotron Radiat. 2022. PMID: 36073871 Free PMC article.
References
-
- Wyman G. M. The Cis-Trans Isomerization of Conjugated Compounds. Chem. Rev. 1955, 55, 625–657. 10.1021/cr50004a001. - DOI
-
- Wyman G. M.; Brode W. R. The Relation between the Absorption Spectra and the Chemical Constitution of Dyes XXII. Cis-Trans Isomerism in Thioindigo Dyes. J. Am. Chem. Soc. 1951, 73, 1487–1493. 10.1021/ja01148a023. - DOI
-
- Brode W. R.; Pearson E. G.; Wyman G. M. The Relation between the Absorption Spectra and the Chemical Constitution of Dyes. XXVII. Cis-Trans Isomerism and Hydrogen Bonding in Indigo Dyes. J. Am. Chem. Soc. 1954, 76, 1034–1036. 10.1021/ja01633a033. - DOI
LinkOut - more resources
Full Text Sources

