Antipruritic Placebo Effects by Conditioning H1-antihistamine
- PMID: 31490841
- PMCID: PMC6844655
- DOI: 10.1097/PSY.0000000000000743
Antipruritic Placebo Effects by Conditioning H1-antihistamine
Abstract
Objective: Allergic rhinitis symptoms can be reduced by behaviorally conditioning antihistamine. It is unclear whether these findings extend to histamine-induced itch or work when participants are informed about the conditioning procedure (open-label conditioning). The current study aims to investigate the efficacy of (open-label) antipruritic behavioral conditioning for histamine-induced itch.
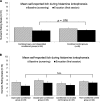
Methods: Healthy participants (n = 92; 84% female) were randomized to I) an open-label conditioned, II) closed-label conditioned, III) conditioned-not-evoked control, or IV) nonconditioned control group. A two-phase conditioning paradigm was used. During acquisition, a conditioned stimulus (CS; distinctively tasting beverage) was repeatedly paired with the H1-antihistamine levocetirizine (groups I-III). During evocation, the CS was paired with placebo (I, II), or instead of the CS, water was paired with placebo (III). The nonconditioned control group (IV) received CS with placebo in both phases. Itch after histamine iontophoresis and physiological data (i.e., spirometry, heart rate, skin conductance) were assessed. Combined conditioned and combined control groups were first compared, and analyses were repeated for separate groups.
Results: Marginally lower itch was reported in the combined conditioned compared with the control groups (F(1,88) = 2.10, p = .076, ηpartial = 0.02); no differences between separate groups were found. No effects on physiological data were found, except for heart rate, which reduced significantly and consistently for control groups, and less consistently for conditioned groups (group by time interaction: F(7,80) = 2.35, p = .031, ηpartial = 0.17).
Conclusion: Limited support was found for the efficacy of antipruritic behavioral conditioning, regardless of whether participants were informed about the conditioning procedure. The application of open-label conditioning in patient populations should be further researched.
Trial registration: www.trialregister.nl; ID NTR5544.
Figures



References
-
- Bartels DJP, van Laarhoven AIM, van de Kerkhof PCM, Evers AWM. Placebo and nocebo effects on itch: effects, mechanisms, and predictors. Eur J Pain 2016;20:8–13. - PubMed
-
- Sölle A, Bartholomäus T, Worm M, Klinger R. How to psychologically minimize scratching impulses. Z Psychol 2014;222:140–7.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

