Tamoxifen suppresses pancreatic β-cell proliferation in mice
- PMID: 31490929
- PMCID: PMC6731016
- DOI: 10.1371/journal.pone.0214829
Tamoxifen suppresses pancreatic β-cell proliferation in mice
Abstract
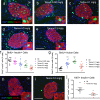
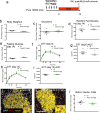
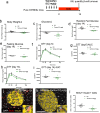
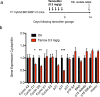
Tamoxifen is a mixed agonist/antagonist estrogen analogue that is frequently used to induce conditional gene deletion in mice using Cre-loxP mediated gene recombination. Tamoxifen is routinely employed in extremely high-doses relative to typical human doses to induce efficient gene deletion in mice. Although tamoxifen has been widely assumed to have no influence upon β-cells, the acute developmental and functional consequences of high-dose tamoxifen upon glucose homeostasis and adult β-cells are largely unknown. We tested if tamoxifen influences glucose homeostasis in male mice of various genetic backgrounds. We then carried out detailed histomorphometry studies of mouse pancreata. We also performed gene expression studies with islets of tamoxifen-treated mice and controls. Tamoxifen had modest effects upon glucose homeostasis of mixed genetic background (F1 B6129SF1/J) mice, with fasting hyperglycemia and improved glucose tolerance but without overt effects on fed glucose levels or insulin sensitivity. Tamoxifen inhibited proliferation of β-cells in a dose-dependent manner, with dramatic reductions in β-cell turnover at the highest dose (decreased by 66%). In sharp contrast, tamoxifen did not reduce proliferation of pancreatic acinar cells. β-cell proliferation was unchanged by tamoxifen in 129S2 mice but was reduced in C57Bl6 genetic background mice (decreased by 59%). Gene expression studies revealed suppression of RNA for cyclins D1 and D2 within islets of tamoxifen-treated mice. Tamoxifen has a cytostatic effect on β-cells, independent of changes in glucose homeostasis, in mixed genetic background and also in C57Bl6 mice. Tamoxifen should be used judiciously to inducibly inactivate genes in studies of glucose homeostasis.
Conflict of interest statement
JAK currently serves as medical director of McNair Interests, a private equity group with investments in type 1 diabetes and other chronic illnesses and is also an advisor for Sanofi and Lexicon. CJL declares no conflict of interest relevant to this article. MMR currently serves as a full time employee of Janssen Research and Development, Johnson and Johnson, Springhouse, PA. AG currently serves as a full time employee of the Novartis Institutes for BioMedical Research (NIBR), a subsidiary of Novartis International AG, Cambridge, MA. No other potential conflicts of interest relevant to this article were reported. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

