Desmoplasia and oncogene driven acinar-to-ductal metaplasia are concurrent events during acinar cell-derived pancreatic cancer initiation in young adult mice
- PMID: 31490946
- PMCID: PMC6731019
- DOI: 10.1371/journal.pone.0221810
Desmoplasia and oncogene driven acinar-to-ductal metaplasia are concurrent events during acinar cell-derived pancreatic cancer initiation in young adult mice
Abstract
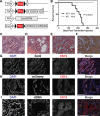
The five-year survival rate of patients diagnosed with advanced pancreatic ductal adenocarcinoma (PDAC) has remained static at <5% despite decades of research. With the exception of erlotinib, clinical trials have failed to demonstrate the benefit of any targeted therapy for PDAC despite promising results in preclinical animal studies. The development of more refined mouse models of PDAC which recapitulate the carcinogenic progression from non-neoplastic, adult exocrine subsets of pancreatic cells to invasive carcinoma in humans are needed to facilitate the accurate translation of therapies to the clinic. To study acinar cell-derived PDAC initiation, we developed a genetically engineered mouse model of PDAC, called KPT, utilizing a tamoxifen-inducible Cre recombinase/estrogen receptor (ESR1) fusion protein knocked into the Ptf1a locus to activate the expression of oncogenic KrasG12D and Trp53R270H alleles in mature pancreatic acinar cells. Oncogene-expressing acinar cells underwent acinar-to-ductal metaplasia, and formed pancreatic intraepithelial neoplasia lesions following the induction of oncogene expression. After a defined latency period, oncogene-expressing acinar cells initiated the formation of highly differentiated and fibrotic tumors, which metastasized to the lungs and liver. Whole-transcriptome analysis of microdissected regions of acinar-to-ductal metaplasia and histological validation experiments demonstrated that regions of acinar-to-ductal metaplasia are characterized by the deposition of the extracellular matrix component hyaluronan. These results indicate that acinar cells expressing KrasG12D and Trp53R270H can initiate PDAC development in young adult mice and implicate hyaluronan deposition in the formation of the earliest characterized PDAC precursor lesions (and the progression of pancreatic cancer). Further studies are necessary to provide a comprehensive characterization of PDAC progression and treatment response in KPT mice and to investigate whether the KPT model could be used as a tool to study translational aspects of acinar cell-derived PDAC tumorigenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Noone AM, Howlader N, Krapcho M, Miller D, Brest A, et al. (2018) SEER Cancer Statistics Review, 1975–2015. Nov. 2017 ed. Bethesda, MD: National Cancer Institute.
-
- Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, et al. (1997) Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 225: 621–633; discussion 633–626. 10.1097/00000658-199705000-00018 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

