Inflammatory biomarker levels over 48 weeks with dual vs triple lopinavir/ritonavir-based therapy: Substudy of a randomized trial
- PMID: 31490959
- PMCID: PMC6730918
- DOI: 10.1371/journal.pone.0221653
Inflammatory biomarker levels over 48 weeks with dual vs triple lopinavir/ritonavir-based therapy: Substudy of a randomized trial
Abstract
Background: Inflammation has been associated with increased morbidity and mortality in HIV-positive patients. We compared inflammatory biomarkers with dual therapy using lopinavir/ritonavir plus lamivudine (LPV/r+3TC) versus triple therapy using LPV/r plus two nucleoside reverse transcriptase inhibitors (LPV/r+2NRTIs) in treatment-naïve HIV-positive adults.
Methods: This was a substudy among Argentinian participants in the randomized trial GARDEL. We measured hsCRP, IL-6, MCP-1, TNF, D-dimer and sCD14 from plasma collected at baseline, week 24 and week 48. Generalized estimating equations with an identity/logit link were used to model the average impact of dual versus triple therapy on each biomarker over time, controlling for baseline levels. Additional models estimated the average effect of virologic suppression on biomarker levels over time, adjusting for age, sex, and baseline CD4 count.
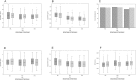
Results: Of 191 trial participants enrolled in Argentina, 172 had baseline and follow-up measurements and were included. Median (IQR) age was 35.5 (28.5, 45) years and CD4 cell count was 310 (219, 414) cells/mm3. Dual therapy was not associated with significantly different biomarker levels over 48 weeks relative to triple therapy. Virologic suppression was associated with statistically significant decreases in MCP-1, TNF and D-dimer levels and an unexpected increase in sCD14 levels. No change was observed in hsCRP or the proportion of participants with undetectable IL-6 levels.
Conclusions: In addition to having virologic non-inferiority, LPV/r+3TC dual therapy is generally associated with similar inflammatory biomarker levels over 48 weeks compared to LPV/r+2NRTIs triple therapy in treatment-naïve adults. Further study of dual treatment regimens is warranted.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: This work was supported by Abbvie and by a grant from the Canadian Institutes of Health Research. DHST is a Site Principal Investigator for clinical trials sponsored by Glaxo Smith Kline, and his institution has received research support for investigator-initiated research studies from Gilead Sciences and Viiv Healthcare outside the submitted work. AG reports grants from Abbvie during the conduct of the study. MWH reports personal fees paid to his institution for advisory boards and speaking engagements from Abbvie, BMS, Gilead, Janssen, Merck, and Viiv outside the submitted work. SW has served on advisory boards, speaking engagements, meetings, symposiums and clinical studies for Viiv Healthcare, Glaxo Smith Kline, Merck and Gilead Sciences outside the submitted work. PC reports grants from Merck, Abbvie, and ViiV healthcare and personal fees from Merck and ViiV healthcare outside the submitted work. All other authors declare no potential conflicts of interest. JR is co-investigator on four projects outside the submitted work with in-kind contributions or financial support from Merck and Gilead Sciences. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. [cited 2016 March 22]. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
-
- Baril JG, Angel JB, Gill MJ, Gathe J, Cahn P, van Wyk J, et al. Dual Therapy Treatment Strategies for the Management of Patients Infected with HIV: A Systematic Review of Current Evidence in ARV-Naive or ARV-Experienced, Virologically Suppressed Patients. PLoS One. 2016;11(2):e0148231 10.1371/journal.pone.0148231 eCollection 2016. - DOI - PMC - PubMed
-
- Cahn P, Andrade-Villanueva J, Arribas JR, Gatell JM, Lama JR, Norton M, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. 2014;14(7):572–80. 10.1016/S1473-3099(14)70736-4 Epub 2014 Apr 27. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous