Psiadia punctulata major flavonoids alleviate exaggerated vasoconstriction produced by advanced glycation end products
- PMID: 31491007
- PMCID: PMC6730914
- DOI: 10.1371/journal.pone.0222101
Psiadia punctulata major flavonoids alleviate exaggerated vasoconstriction produced by advanced glycation end products
Abstract
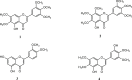
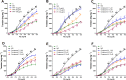
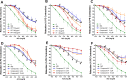
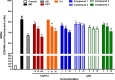
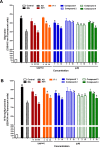
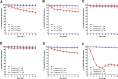
Exaggerated vasoconstriction plays important roles in vascular complication in aging and many diseases like diabetes. Here, we investigated the protective effect of Psiadia punctulata (PP) on advanced glycation end products (AGEs)-induced aggravated vasoconstriction. The effect of total methanol extract of PP leaves (PPT) on AGE-induced vascular injury was studied through bioassay-guided fractionation procedures in order to find the bioactive fraction and isolate the bioactive compounds. Vascular reactivity was studied using the isolated artery technique by adding cumulative concentrations of phenylephrine (PE) or acetyl choline (ACh). In addition, the antiglycating effect, as well as the effect on AGEs intermediates dityrosine and N`-formylkynurenine and their radical scavenging activity were measured. The results showed that PPT alleviated the AGEs-induced aggravated vasoconstriction in a concentration-dependent manner. The bioassay guided fractionation procedures suggested the chloroform fraction (Fr I) to be responsible for the activity. Chemical investigation of this fraction resulted in isolation of four major bioactive compounds that were identified as: umuhengerin (1), gardenin (2), luteolin-3`,4`-dimethyl ether (3), and 5,3`-dihydroxy-6,7,4`,5`-tetramethoxyflavone (4). The four compounds alleviated the exaggerated vasoconstriction in a dose dependent manner. In search for their mechanism of action, we observed that PPT, Fr. I and the isolated compounds did not improve the impaired vasodilation associated with AGEs exposure. PPT, Fr. I and the isolated compounds 1-4 inhibited AGEs formation and their protein oxidation intermediates. Furthermore, PPT, Fr. I and the isolated compounds 1-4 showed weak radical scavenging activity with compound 4 as the most potent. In conclusion, PPT appears to protect against AGEs-induced exaggerated vasoconstriction through antiglycation and radical scavenging activities.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

