Possible roles of monocytes/macrophages in response to elephant endotheliotropic herpesvirus (EEHV) infections in Asian elephants (Elephas maximus)
- PMID: 31491031
- PMCID: PMC6730851
- DOI: 10.1371/journal.pone.0222158
Possible roles of monocytes/macrophages in response to elephant endotheliotropic herpesvirus (EEHV) infections in Asian elephants (Elephas maximus)
Abstract
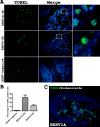
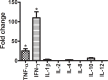
Elephant endotheliotropic herpesvirus-hemorrhagic disease (EEHV-HD) is the primary cause of acute, highly fatal, hemorrhagic diseases in young Asian elephants. Although monocytopenia is frequently observed in EEHV-HD cases, the role monocytes play in EEHV-disease pathogenesis is unknown. This study seeks to explain the responses of monocytes/macrophages in the pathogenesis of EEHV-HD. Samples of blood, frozen tissues, and formalin-fixed, paraffin-embedded (FFPE) tissues from EEHV1A-HD, EEHV4-HD, co-infected EEHV1A and 4-HD, and EEHV-negative calves were analyzed. Peripheral blood mononuclear cells (PBMCs) from the persistent EEHV4-infected and EEHV-negative calves were also studied. The results showed increased infiltration of Iba-1-positive macrophages in the inflamed tissues of the internal organs of elephant calves with EEHV-HD. In addition, cellular apoptosis also increased in the tissues of elephants with EEHV-HD, especially in the PBMCs, compared to the EEHV-negative control. In the PBMCs of persistent EEHV4-infected elephants, cytokine mRNA expression was high, particularly up-regulation of TNF-α and IFN-γ. Moreover, viral particles were observed in the cytoplasm of the persistent EEHV4-infected elephant monocytes. Our study demonstrated for the first time that apoptosis of the PBMCs increased in cases of EEHV-HD. Furthermore, this study showed that monocytes may serve as a vehicle for viral dissemination during EEHV infection in Asian elephants.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Wilkie GS, Davison AJ, Kerr K, Stidworthy MF, Redrobe S, Steinbach F, et al. First fatality associated with elephant endotheliotropic herpesvirus 5 in an Asian elephant: pathological findings and complete viral genome sequence. Scientific Reports. 2014. September 09;4:6299 10.1038/srep06299 - DOI - PMC - PubMed
-
- Richman LK, Zong JC, Latimer EM, Lock J, Fleischer RC, Heaggans SY, et al. Elephant endotheliotropic herpesviruses EEHV1A, EEHV1B, and EEHV2 from cases of hemorrhagic disease are highly diverged from other mammalian herpesviruses and may form a new subfamily. Journal of Virology. 2014. December;88(23):13523–46. 10.1128/JVI.01673-14 - DOI - PMC - PubMed
-
- Dastjerdi A, Seilern-Moy K, Darpel K, Steinbach F, Molenaar F. Surviving and fatal Elephant Endotheliotropic Herpesvirus-1A infections in juvenile Asian elephants—lessons learned and recommendations on anti-herpesviral therapy. BMC Veterinary Research. 2016. August 27;12(1):178 10.1186/s12917-016-0806-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

