A Molecular Dynamics Study of Vasoactive Intestinal Peptide Receptor 1 and the Basis of Its Therapeutic Antagonism
- PMID: 31491880
- PMCID: PMC6770453
- DOI: 10.3390/ijms20184348
A Molecular Dynamics Study of Vasoactive Intestinal Peptide Receptor 1 and the Basis of Its Therapeutic Antagonism
Abstract
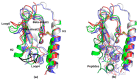
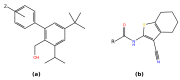
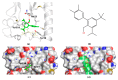
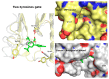
Vasoactive intestinal peptide receptor 1 (VPAC1) is a member of a secretin-like subfamily of G protein-coupled receptors. Its endogenous neuropeptide (VIP), secreted by neurons and immune cells, modulates various physiological functions such as exocrine and endocrine secretions, immune response, smooth muscles relaxation, vasodilation, and fetal development. As a drug target, VPAC1 has been selected for therapy of inflammatory diseases but drug discovery is still hampered by lack of its crystal structure. In this study we presented the homology model of this receptor constructed with the well-known web service GPCRM. The VPAC1 model is composed of extracellular and transmembrane domains that form a complex with an endogenous hormone VIP. Using the homology model of VPAC1 the mechanism of action of potential drug candidates for VPAC1 was described. Only two series of small-molecule antagonists of confirmed biological activity for VPAC1 have been described thus far. Molecular docking and a series of molecular dynamics simulations were performed to elucidate their binding to VPAC1 and resulting antagonist effect. The presented work provides the basis for the possible binding mode of VPAC1 antagonists and determinants of their molecular recognition in the context of other class B GPCRs. Until the crystal structure of VPAC1 will be released, the presented homology model of VPAC1 can serve as a scaffold for drug discovery studies and is available from the author upon request.
Keywords: G protein-coupled receptors; GPCR activation; GPCRM; PACAP; VIP; VIPR1; VPAC1; agonist; antagonist; gut hormone receptors; homology modeling; molecular dynamics; vasoactive intestinal peptide receptor 1.
Conflict of interest statement
The author declares no conflict of interest.
Figures

Similar articles
-
Drug Repositioning For Allosteric Modulation of VIP and PACAP Receptors.Front Endocrinol (Lausanne). 2021 Nov 18;12:711906. doi: 10.3389/fendo.2021.711906. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34867774 Free PMC article.
-
VPAC1 receptor binding site: contribution of photoaffinity labeling approach.Neuropeptides. 2010 Apr;44(2):127-32. doi: 10.1016/j.npep.2009.11.008. Epub 2009 Dec 23. Neuropeptides. 2010. PMID: 20031208 Review.
-
VPAC receptors: structure, molecular pharmacology and interaction with accessory proteins.Br J Pharmacol. 2012 May;166(1):42-50. doi: 10.1111/j.1476-5381.2011.01676.x. Br J Pharmacol. 2012. PMID: 21951273 Free PMC article. Review.
-
A cloned frog vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating polypeptide receptor exhibits pharmacological and tissue distribution characteristics of both VPAC1 and VPAC2 receptors in mammals.Endocrinology. 1999 Mar;140(3):1285-93. doi: 10.1210/endo.140.3.6576. Endocrinology. 1999. PMID: 10067855
-
Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology.Peptides. 2007 Sep;28(9):1631-9. doi: 10.1016/j.peptides.2007.04.026. Epub 2007 May 22. Peptides. 2007. PMID: 17574305 Review.
Cited by
-
Drug Repositioning For Allosteric Modulation of VIP and PACAP Receptors.Front Endocrinol (Lausanne). 2021 Nov 18;12:711906. doi: 10.3389/fendo.2021.711906. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34867774 Free PMC article.
-
Targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors.Int J Mol Sci. 2022 Jul 22;23(15):8069. doi: 10.3390/ijms23158069. Int J Mol Sci. 2022. PMID: 35897648 Free PMC article. Review.
-
TAGOPSIN: collating taxa-specific gene and protein functional and structural information.BMC Bioinformatics. 2021 Oct 23;22(1):517. doi: 10.1186/s12859-021-04429-5. BMC Bioinformatics. 2021. PMID: 34688246 Free PMC article.
-
Helix 8 in chemotactic receptors of the complement system.PLoS Comput Biol. 2022 Jul 21;18(7):e1009994. doi: 10.1371/journal.pcbi.1009994. eCollection 2022 Jul. PLoS Comput Biol. 2022. PMID: 35862436 Free PMC article.
-
Signal Transduction by VIP and PACAP Receptors.Biomedicines. 2022 Feb 9;10(2):406. doi: 10.3390/biomedicines10020406. Biomedicines. 2022. PMID: 35203615 Free PMC article. Review.
References
-
- Harmar A.J., Arimura A., Gozes I., Journot L., Laburthe M., Pisegna J.R., Rawlings S.R., Robberecht P., Said S.I., Sreedharan S.P., et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998;50:265–270. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

