Twenty Years of Progress Toward West Nile Virus Vaccine Development
- PMID: 31491885
- PMCID: PMC6784102
- DOI: 10.3390/v11090823
Twenty Years of Progress Toward West Nile Virus Vaccine Development
Abstract
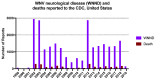
Although West Nile virus (WNV) has been a prominent mosquito-transmitted infection in North America for twenty years, no human vaccine has been licensed. With a cumulative number of 24,714 neurological disease cases and 2314 deaths in the U.S. since 1999, plus a large outbreak in Europe in 2018 involving over 2000 human cases in 15 countries, a vaccine is essential to prevent continued morbidity, mortality, and economic burden. Currently, four veterinary vaccines are licensed, and six vaccines have progressed into clinical trials in humans. All four veterinary vaccines require multiple primary doses and annual boosters, but for a human vaccine to be protective and cost effective in the most vulnerable older age population, it is ideal that the vaccine be strongly immunogenic with only a single dose and without subsequent annual boosters. Of six human vaccine candidates, the two live, attenuated vaccines were the only ones that elicited strong immunity after a single dose. As none of these candidates have yet progressed beyond phase II clinical trials, development of new candidate vaccines and improvement of vaccination strategies remains an important area of research.
Keywords: West Nile virus; flavivirus; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Smithburn K.C., Hughes T.P., Burke A.W., Paul J.H. A neurotropic virus isolated from the blood of a native of uganda. Am. J. Trop. Med. Hyg. 1940;20:471–492. doi: 10.4269/ajtmh.1940.s1-20.471. - DOI
-
- West Nile Virus. [(accessed on 5 July 2019)]; Available online: https://www.cdc.gov/westnile/index.html.
-
- West Nile Virus—Symptoms, Diagnosis, & Treatment. [(accessed on 5 July 2019)]; Available online: https://www.cdc.gov/westnile/symptoms/index.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

