Efficacy of 3D Culture Priming is Maintained in Human Mesenchymal Stem Cells after Extensive Expansion of the Cells
- PMID: 31491901
- PMCID: PMC6770505
- DOI: 10.3390/cells8091031
Efficacy of 3D Culture Priming is Maintained in Human Mesenchymal Stem Cells after Extensive Expansion of the Cells
Abstract
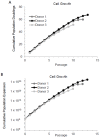
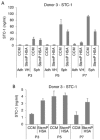
The use of non-optimal preparations of mesenchymal stem cells (MSCs), such as extensively expanded cells, might be necessary to obtain the large numbers of cells needed for many clinical applications. We previously demonstrated that minimally expanded (early passage) MSCs can be pre-activated as spheroids to produce potentially therapeutic factors in 3D cultures. Here, we used extensively expanded (late passage) MSCs and studied their 3D-culture activation potential. MSCs were culture-expanded as 2D monolayers, and cells from various passages were activated by 3D culture in hanging drops with either fetal bovine serum (FBS)-containing media or a more clinically-applicable animal product-free (xeno-free) media. Gene expression analyses demonstrated that MSC spheroids prepared from passage 3, 5, and 7 cells were similar to each other but different from 2D MSCs. Furthermore, the expression of notable anti-inflammatory/immune-modulatory factors cyclooxygenase-2 (PTGS2), TNF alpha induced protein 6 (TNFAIP6), and stanniocalcin 1 (STC-1) were up-regulated in all spheroid preparations. This was confirmed by the detection of secreted prostaglandin E2 (PGE-2), tumor necrosis factor-stimulated gene 6 (TSG-6, and STC-1. This study demonstrated that extensively expanded MSCs can be activated in 3D culture through spheroid formation in both FBS-containing and xeno-free media. This work highlights the possibility of activating otherwise less useable MSC preparations through 3D culture generating large numbers of potentially therapeutic MSCs.
Keywords: 3D; MSC; PGE2; TSG-6; anti-inflammatory; culture-expansion; immunomodulatory; passage; spheroid; xeno-free.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





Similar articles
-
Production and Administration of Therapeutic Mesenchymal Stem/Stromal Cell (MSC) Spheroids Primed in 3-D Cultures Under Xeno-free Conditions.J Vis Exp. 2017 Mar 18;(121):55126. doi: 10.3791/55126. J Vis Exp. 2017. PMID: 28362380 Free PMC article.
-
Unique characteristics of human mesenchymal stromal/progenitor cells pre-activated in 3-dimensional cultures under different conditions.Cytotherapy. 2014 Nov;16(11):1486-1500. doi: 10.1016/j.jcyt.2014.07.010. Epub 2014 Sep 16. Cytotherapy. 2014. PMID: 25231893 Free PMC article.
-
Changes in the secretome of tri-dimensional spheroid-cultured human mesenchymal stem cells in vitro by interleukin-1 priming.Stem Cell Res Ther. 2018 Jan 17;9(1):11. doi: 10.1186/s13287-017-0753-5. Stem Cell Res Ther. 2018. PMID: 29343288 Free PMC article.
-
Influences of Xeno-Free Media on Mesenchymal Stem Cell Expansion for Clinical Application.Tissue Eng Regen Med. 2021 Feb;18(1):15-23. doi: 10.1007/s13770-020-00306-z. Epub 2020 Nov 4. Tissue Eng Regen Med. 2021. PMID: 33150562 Free PMC article. Review.
-
Specificity of 3D MSC Spheroids Microenvironment: Impact on MSC Behavior and Properties.Stem Cell Rev Rep. 2020 Oct;16(5):853-875. doi: 10.1007/s12015-020-10006-9. Stem Cell Rev Rep. 2020. PMID: 32681232 Review.
Cited by
-
Age-Related Changes in the Inflammatory Status of Human Mesenchymal Stem Cells: Implications for Cell Therapy.Stem Cell Reports. 2021 Apr 13;16(4):694-707. doi: 10.1016/j.stemcr.2021.01.021. Epub 2021 Feb 25. Stem Cell Reports. 2021. PMID: 33636113 Free PMC article. Review.
-
Current perspectives on the dynamic culture of mesenchymal stromal/stem cell spheroids.Stem Cells Transl Med. 2025 Mar 18;14(3):szae093. doi: 10.1093/stcltm/szae093. Stem Cells Transl Med. 2025. PMID: 39737878 Free PMC article. Review.
-
A comparative study of mesenchymal stem cells cultured as cell-only aggregates and in encapsulated hydrogels.J Tissue Eng Regen Med. 2022 Jan;16(1):14-25. doi: 10.1002/term.3257. Epub 2021 Oct 22. J Tissue Eng Regen Med. 2022. PMID: 34655456 Free PMC article.
-
Dual Bioelectrical Assessment of Human Mesenchymal Stem Cells Using Plasma and Mitochondrial Membrane Potentiometric Probes.Bioelectricity. 2020 Sep 1;2(3):238-250. doi: 10.1089/bioe.2020.0006. Epub 2020 Sep 16. Bioelectricity. 2020. PMID: 34476356 Free PMC article.
-
Xenobiotic-Free Medium Guarantees Expansion of Adipose Tissue-Derived Canine Mesenchymal Stem Cells Both in 3D Fibrin-Based Matrices and in 2D Plastic Surface Cultures.Cells. 2020 Dec 2;9(12):2578. doi: 10.3390/cells9122578. Cells. 2020. PMID: 33276432 Free PMC article.
References
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

