Decreased Level of Neurotrophic Factor Neuritin 1 in Women with Ovarian Endometriosis after Receiving Gonadotropin-Releasing Hormone Agonist Treatment
- PMID: 31491902
- PMCID: PMC6770869
- DOI: 10.3390/ijms20184352
Decreased Level of Neurotrophic Factor Neuritin 1 in Women with Ovarian Endometriosis after Receiving Gonadotropin-Releasing Hormone Agonist Treatment
Abstract
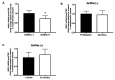
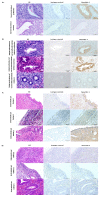
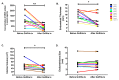
This study aimed to investigate the effect of gonadotropin-releasing hormone agonist (GnRHa) treatment on the expression of neuritin 1 (NRN1) in women with ovarian endometriosis. We collected tissues and serum from women with endometriosis treated with (n = 45) or without (n = 37) GnRHa. NRN1 mRNA and protein levels were measured using qPCR and Western blot. Immunolocalization of NRN1 in endometriotic tissues was examined using immunohistochemistry. In addition, a follow-up study was carried out to monitor the serum level of NRN1 in patients before and after GnRHa treatment. Both mRNA (p = 0.046) and protein (p = 0.0155) levels of NRN1 were significantly lower in endometriotic tissues from patients receiving GnRHa treatment compared to the untreated group. Both epithelial and stromal cells of endometriotic tissues from untreated women with endometriosis exhibited stronger staining of NRN1 but not in those who were treated with GnRHa. The follow-up study showed that the serum level of the NRN1 concentration decreased significantly from 1149 ± 192.3 to 379.2 ± 80.16 pg/mL after GnRHa treatment (p = 0.0098). The expression of NRN1 was significantly lower in women with ovarian endometriosis treated with GnRHa. These results suggest that NRN1 may be a biomarker response to the effect of GnRHa treatment for patients with ovarian endometriosis.
Keywords: GnRHa; NRN1; gonadotropin-releasing hormone agonist; neuritin 1; ovarian endometriosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Gonadotropin-releasing hormone agonist induces downregulation of tensin 1 in women with endometriosis.Acta Obstet Gynecol Scand. 2019 Feb;98(2):222-231. doi: 10.1111/aogs.13481. Epub 2018 Nov 11. Acta Obstet Gynecol Scand. 2019. PMID: 30312486
-
Tumor necrosis factor-alpha-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-kappa B activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression.J Clin Endocrinol Metab. 2003 Feb;88(2):730-5. doi: 10.1210/jc.2002-020666. J Clin Endocrinol Metab. 2003. PMID: 12574206
-
Effect of GnRH agonist therapy on the expression of human heat shock protein 70 in eutopic and ectopic endometria of women with endometriosis.Eur J Obstet Gynecol Reprod Biol. 2014 Sep;180:16-23. doi: 10.1016/j.ejogrb.2014.06.002. Epub 2014 Jun 27. Eur J Obstet Gynecol Reprod Biol. 2014. PMID: 25016551
-
Gonadotropin-releasing hormone and reproductive medicine.J Obstet Gynaecol Can. 2003 Feb;25(2):98-113. doi: 10.1016/s1701-2163(16)30206-7. J Obstet Gynaecol Can. 2003. PMID: 12577127 Review.
-
The use of gonadotropin releasing hormone analogues in adolescent and young patients with endometriosis.Curr Opin Obstet Gynecol. 2013 Aug;25(4):287-92. doi: 10.1097/GCO.0b013e32836343eb. Curr Opin Obstet Gynecol. 2013. PMID: 23770813 Review.
Cited by
-
Exposure to gonadotropin-releasing hormone agonist in early pregnancy leads to adverse pregnancy outcomes: a retrospective analysis.Arch Gynecol Obstet. 2025 Mar;311(3):801-809. doi: 10.1007/s00404-024-07914-3. Epub 2025 Feb 13. Arch Gynecol Obstet. 2025. PMID: 39945791 Free PMC article.
-
Salvia miltiorrhiza-Containing Chinese Herbal Medicine Combined With GnRH Agonist for Postoperative Treatment of Endometriosis: A Systematic Review and meta-Analysis.Front Pharmacol. 2022 Feb 16;13:831850. doi: 10.3389/fphar.2022.831850. eCollection 2022. Front Pharmacol. 2022. PMID: 35250579 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 99-2632-B-038-001-MY3/National Science Council
- BM102020946; BM103010097; BM104010117; BM10501010036; BM10601010024; BM10701010027/Academia Sinica
- MG-105-SP-07; MG-106-SP-07; MG-107-SP-06/National Health Research Institutes
- MOST-104-2314-B-038-063-MY2; 106-2314-B-038-072; 107-2314-B-038-006/Ministry of Science and Technology
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

