First Results from a Screening of 300 Naturally Occurring Compounds: 4,6-dibromo-2-(2',4'-dibromophenoxy)phenol, 4,5,6-tribromo-2-(2',4'-dibromophenoxy)phenol, and 5-epi-nakijinone Q as Substances with the Potential for Anticancer Therapy
- PMID: 31491907
- PMCID: PMC6780284
- DOI: 10.3390/md17090521
First Results from a Screening of 300 Naturally Occurring Compounds: 4,6-dibromo-2-(2',4'-dibromophenoxy)phenol, 4,5,6-tribromo-2-(2',4'-dibromophenoxy)phenol, and 5-epi-nakijinone Q as Substances with the Potential for Anticancer Therapy
Abstract
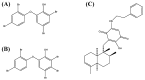
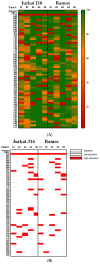
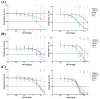
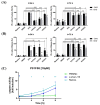
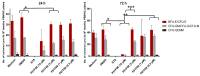
There is a variety of antineoplastic drugs that are based on natural compounds from ecological niches with high evolutionary pressure. We used two cell lines (Jurkat J16 and Ramos) in a screening to assess 300 different naturally occurring compounds with regard to their antineoplastic activity. The results of the compounds 4,6-dibromo-2-(2',4'-dibromophenoxy)phenol (P01F03), 4,5,6-tribromo-2-(2',4'-dibromophenoxy)phenol (P01F08), and 5-epi-nakijinone Q (P03F03) prompted us to perform further research. Using viability and apoptosis assays on the cell lines of primary human leukemic and normal hematopoietic cells, we found that P01F08 induced apoptosis in the cell lines at IC50 values between 1.61 and 2.95 μM after 72 h. IC50 values of peripheral blood mononuclear cells (PBMNCs) from healthy donors were higher, demonstrating that the cytotoxicity in the cell lines reached 50%, while normal PBMNCs were hardly affected. The colony-forming unit assay showed that the hematopoietic progenitor cells were not significantly affected in their growth by P01F08 at a concentration of 3 μM. P01F08 showed a 3.2-fold lower IC50 value in primary leukemic cells [acute myeloid leukemia (AML)] compared to the PBMNC of healthy donors. We could confirm the antineoplastic effect of 5-epi-nakijinone Q (P03F03) on the cell lines via the induction of apoptosis but noted a similarly strong cytotoxic effect on normal PBMNCs.
Keywords: acute myeloid leukemia; apoptosis; bioactive natural products; cytotoxic activity; drug leads; marine sponge derived natural products; peripheral blood mononuclear cells; polybrominated diphenyl ethers; primary leukemic cells; sesquiterpene aminoquinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

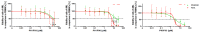
References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C., Akinyemiju T.F., Al Lami F.H., Alam T., Alizadeh-Navaei R., Allen C., Alsharif U., Alvis-Guzman N., Amini E., et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4:1553–1568. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

