Glucagon-Like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes: Prescription According to Reimbursement Constraints and Guideline Recommendations in Catalonia
- PMID: 31491916
- PMCID: PMC6780172
- DOI: 10.3390/jcm8091389
Glucagon-Like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes: Prescription According to Reimbursement Constraints and Guideline Recommendations in Catalonia
Abstract
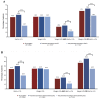
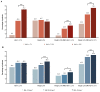
To assess the clinical characteristics, the prescription pattern of GLP-1 receptor agonists (GLP-1RA) users, and HbA1c and weight change, we retrospectively assessed patients with type 2 diabetes by initiating GLP-1RA as an add-on to the standard of care in Catalonia. The mean change from the baseline in glycated hemoglobin (HbA1c) and weight at 6 and 12 months of therapy was calculated, and we assessed the predictors of the HbA1c reduction of ≥1% and/or the weight reduction of ≥3% as recommended by the Catalan Health Service. In 2854 patients who initiated a GLP-1RA during 2014 and 2015, the overall mean HbA1c values were reduced from the baseline by -0.84% (SD = 1.66) (-9.2 mmol/mol) and lost on average 2.73 kg (SD = 6.2). About 44% percent of patients decreased their HbA1c by ≥1%; 44% decreased their weight by ≥3%; and only 22% met both of them together. The odds of achieving a reduction of ≥1% in initial HbA1c were two-fold higher for patients with higher baseline levels, and the likelihood of a reduction of ≥3% in the initial weight was associated with a higher BMI at the baseline, but they were independent of each other. The composite outcome (target 1% HbA1c reduction and 3% weight loss) to evaluate both the GLP-1RA clinical benefit and treatment withdrawal should be judged from a patient-centered approach.
Keywords: GLP-1 analogue; glycaemic control; observational study; primary care; type 2 diabetes mellitus.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. M. M.-C. has received an advisory honorarium from Astra-Zeneca, Bayer, Boehringer Ingelheim, GSK, Lilly, MSD, Novartis, Novo Nordisk, and Sanofi; he has received a speaker honorarium from Astra-Zeneca, Bayer, Boehringer Ingelheim, GSK, Lilly, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi; he has received research grants to the institution from Astra-Zeneca, GSK, Lilly, MSD, Novartis, Novo Nordisk, and Sanofi. J. F.-N. has received advisory and or speaking fees from Astra-Zeneca, Ascensia, Boehringer Ingelheim, GSK, Lilly, MSD, Novartis, Novo Nordisk, and Sanofi; he has received research grants to the institution from Astra-Zeneca, GSK, Lilly, MSD, Novartis, Novo Nordisk, Sanofi, and Boehringer. D.M. has received advisory and/or speaking fees from Astra-Zeneca, Ascensia, Boehringer Ingelheim, GSK, Lilly, MSD, Novartis, Novo Nordisk, and Sanofi; he has received research grants to the institution from Astra-Zeneca, GSK, Lilly, MSD, Novartis, Novo Nordisk, Sanofi, and Boehringer. E.O. has received advisory and or speaking fees from Astra-Zeneca, Boehringer Ingelheim, Lilly, MSD, Novo Nordisk, Sanofi, and Amgen; he has received research grants to the institution from MSD and Amgen. J.R., B.V., JA.V. and M.G. have no conflict of interest to declare.
Figures
References
-
- Garber A.J., Abrahamson M.J., Barzilay J.I., Blonde L., Bloomgarden Z.T., Bush M.A., Dagogo-Jack S., DeFronzo R.A., Einhorn D., Fonseca V.A., et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm--2016 Executive Summary. Endocr. Pract. 2016;22:84–113. doi: 10.4158/EP151126.CS. - DOI - PubMed
-
- Davies M.J., D’Alessio D.A., Fradkin J., Kernan W.N., Mathieu C., Mingrone G., Rossing P., Tsapas A., Wexler D.J., Buse J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018 doi: 10.2337/dci18-0033. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

