Dual role of Endoplasmic Reticulum Stress-Mediated Unfolded Protein Response Signaling Pathway in Carcinogenesis
- PMID: 31491919
- PMCID: PMC6770252
- DOI: 10.3390/ijms20184354
Dual role of Endoplasmic Reticulum Stress-Mediated Unfolded Protein Response Signaling Pathway in Carcinogenesis
Abstract
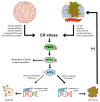
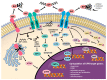
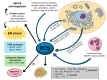
Cancer constitutes a grave problem nowadays in view of the fact that it has become one of the main causes of death worldwide. Poor clinical prognosis is presumably due to cancer cells metabolism as tumor microenvironment is affected by oxidative stress. This event triggers adequate cellular response and thereby creates appropriate conditions for further cancer progression. Endoplasmic reticulum (ER) stress occurs when the balance between an ability of the ER to fold and transfer proteins and the degradation of the misfolded ones become distorted. Since ER is an organelle relatively sensitive to oxidative damage, aforementioned conditions swiftly cause the activation of the unfolded protein response (UPR) signaling pathway. The output of the UPR, depending on numerous factors, may vary and switch between the pro-survival and the pro-apoptotic branch, and hence it displays opposing effects in deciding the fate of the cancer cell. The role of UPR-related proteins in tumorigenesis, such as binding the immunoglobulin protein (BiP) and inositol-requiring enzyme-1α (IRE1α), activating transcription factor 6 (ATF6) or the protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), has already been specifically described so far. Nevertheless, due to the paradoxical outcomes of the UPR activation as well as gaps in current knowledge, it still needs to be further investigated. Herein we would like to elicit the actual link between neoplastic diseases and the UPR signaling pathway, considering its major branches and discussing its potential use in the development of a novel, anti-cancer, targeted therapy.
Keywords: apoptosis; cancer; cancer treatment; carcinogenesis; endoplasmic reticulum stress; protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK); reactive oxygen species; unfolded protein response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mechanism of the induction of endoplasmic reticulum stress by the anti-cancer agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT): Activation of PERK/eIF2α, IRE1α, ATF6 and calmodulin kinase.Biochem Pharmacol. 2016 Jun 1;109:27-47. doi: 10.1016/j.bcp.2016.04.001. Epub 2016 Apr 6. Biochem Pharmacol. 2016. PMID: 27059255
-
The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress.Curr Mol Med. 2016;16(6):533-44. doi: 10.2174/1566524016666160523143937. Curr Mol Med. 2016. PMID: 27211800 Free PMC article. Review.
-
Molecular signal networks and regulating mechanisms of the unfolded protein response.J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review.
-
The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress.Biol Cell. 2012 May;104(5):259-70. doi: 10.1111/boc.201100055. Epub 2012 Feb 29. Biol Cell. 2012. PMID: 22268789 Review.
-
The Role of ER Stress and the Unfolded Protein Response in Cancer.Cancer Genomics Proteomics. 2025 May-Jun;22(3):363-381. doi: 10.21873/cgp.20507. Cancer Genomics Proteomics. 2025. PMID: 40280715 Free PMC article. Review.
Cited by
-
Could the Propionic Acid Treatment in Combination with Metformin be Safe for the Small Intestine of Diabetic Rats?Endocr Metab Immune Disord Drug Targets. 2024;24(11):1335-1345. doi: 10.2174/0118715303273125231121062111. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 38265384
-
Endoplasmic Reticulum Stress and Tumor Microenvironment in Bladder Cancer: The Missing Link.Front Cell Dev Biol. 2021 May 31;9:683940. doi: 10.3389/fcell.2021.683940. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34136492 Free PMC article. Review.
-
Increasing Stress to Induce Apoptosis in Pancreatic Cancer via the Unfolded Protein Response (UPR).Int J Mol Sci. 2022 Dec 29;24(1):577. doi: 10.3390/ijms24010577. Int J Mol Sci. 2022. PMID: 36614019 Free PMC article. Review.
-
The PERK-Dependent Molecular Mechanisms as a Novel Therapeutic Target for Neurodegenerative Diseases.Int J Mol Sci. 2020 Mar 19;21(6):2108. doi: 10.3390/ijms21062108. Int J Mol Sci. 2020. PMID: 32204380 Free PMC article. Review.
-
Hederagenin promotes SIRT6 to attenuate epidural scar formation by aggravating PRMT1 deacetylation.Bone Joint Res. 2025 Jun 13;14(6):516-526. doi: 10.1302/2046-3758.146.BJR-2024-0287.R2. Bone Joint Res. 2025. PMID: 40511498 Free PMC article.
References
-
- Autore F., Sora F., Chiusolo P., Annunziata M., Iurlo A., Cattaneo D., Galimberti S., Bajer J.A., Sica S. ‘Secondary chronic myeloid leukemia’: Comparison between patients previously exposed or not to chemo- and/or radiotherapy. Leuk. Lymphoma. 2019:1–3. doi: 10.1080/10428194.2019.1639162. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

