Tobramycin Promotes Melanogenesis by Upregulating p38 MAPK Protein Phosphorylation in B16F10 Melanoma Cells
- PMID: 31491963
- PMCID: PMC6783951
- DOI: 10.3390/antibiotics8030140
Tobramycin Promotes Melanogenesis by Upregulating p38 MAPK Protein Phosphorylation in B16F10 Melanoma Cells
Abstract
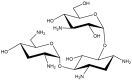
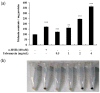
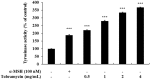
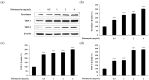
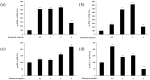
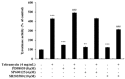
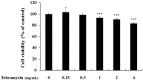
Tobramycin is an aminoglycoside-based natural antibiotic derived from Streptomyces tenebrarius, which is primarily used for Gram-negative bacterial infection treatment. Although tobramycin has been utilized in clinical practice for a long time, it has exhibited several side effects, leading to the introduction of more effective antibiotics. Therefore, we conducted our experiments focusing on new possibilities for the clinical use of tobramycin. How tobramycin affects skin melanin formation is unknown. This study used B16F10 melanoma cells to assess the effect of tobramycin on melanin production. After cytotoxicity was assessed by MTT assay, melanin content and tyrosinase activity analyses revealed that tobramycin induces melanin synthesis in B16F10 cells. Next, Western blot analyses were performed to elucidate the mechanism by which tobramycin increases melanin production; phosphorylated p38 protein expression was upregulated. Protein inhibitors have been used to elucidate the mechanism of tobramycin. Kanamycin A and B are structurally similar to tobramycin, and 2-DOS represents the central structure of these antibiotics. The effects of these substances on melanogenesis were evaluated. Kanamycin A reduced melanin production, whereas kanamycin B and 2-DOS had no effect. Overall, our data indicated that tobramycin increases melanin production by promoting p38 protein phosphorylation in B16F10 melanoma cells.
Keywords: 2-DOS; B16F10 melanoma cell; aminoglycoside; kanamycin; melanin; melanogenesis; p-p38; tobramycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




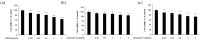
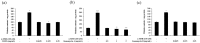

References
LinkOut - more resources
Full Text Sources

