Stability and Catalase-Like Activity of a Mononuclear Non-Heme Oxoiron(IV) Complex in Aqueous Solution
- PMID: 31491998
- PMCID: PMC6766873
- DOI: 10.3390/molecules24183236
Stability and Catalase-Like Activity of a Mononuclear Non-Heme Oxoiron(IV) Complex in Aqueous Solution
Abstract
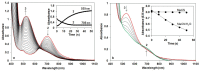
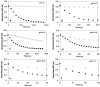
Heme-type catalase is a class of oxidoreductase enzymes responsible for the biological defense against oxidative damage of cellular components caused by hydrogen peroxide, where metal-oxo species are proposed as reactive intermediates. To get more insight into the mechanism of this curious reaction a non-heme structural and functional model was carried out by the use of a mononuclear complex [FeII(N4Py*)(CH3CN)](CF3SO3)2 (N4Py* = N,N-bis(2-pyridylmethyl)- 1,2-di(2-pyridyl)ethylamine) as a catalyst, where the possible reactive intermediates, high-valent FeIV=O and FeIII-OOH are known and spectroscopically well characterized. The kinetics of the dismutation of H2O2 into O2 and H2O was investigated in buffered water, where the reactivity of the catalyst was markedly influenced by the pH, and it revealed Michaelis-Menten behavior with KM = 1.39 M, kcat = 33 s-1 and k2(kcat/KM) = 23.9 M-1s-1 at pH 9.5. A mononuclear [(N4Py)FeIV=O]2+ as a possible intermediate was also prepared, and the pH dependence of its stability and reactivity in aqueous solution against H2O2 was also investigated. Based on detailed kinetic, and mechanistic studies (pH dependence, solvent isotope effect (SIE) of 6.2 and the saturation kinetics for the initial rates versus the H2O2 concentration with KM = 18 mM) lead to the conclusion that the rate-determining step in these reactions above involves hydrogen-atom transfer between the iron-bound substrate and the Fe(IV)-oxo species.
Keywords: catalase activity; hydrogen peroxide; iron(IV)-oxo; kinetic studies; oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

