Nucleoporin 62-Like Protein is Required for the Development of Pharyngeal Arches through Regulation of Wnt/β-Catenin Signaling and Apoptotic Homeostasis in Zebrafish
- PMID: 31492028
- PMCID: PMC6770318
- DOI: 10.3390/cells8091038
Nucleoporin 62-Like Protein is Required for the Development of Pharyngeal Arches through Regulation of Wnt/β-Catenin Signaling and Apoptotic Homeostasis in Zebrafish
Abstract
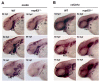
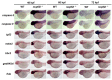
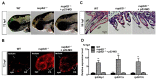
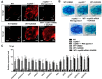
We have previously observed the predominant expression of nucleoporin 62-like (Nup62l) mRNA in the pharyngeal region of zebrafish, which raises the question whether Nup62l has important implications in governing the morphogenesis of pharyngeal arches (PA) in zebrafish. Herein, we explored the functions of Nup62l in PA development. The disruption of Nup62l with a CRISPR/Cas9-dependent gene knockout approach led to defective PA, which was characterized by a thinned and shortened pharyngeal region and a significant loss of pharyngeal cartilages. During pharyngeal cartilage formation, prechondrogenic condensation and chondrogenic differentiation were disrupted in homozygous nup62l-mutants, while the specification and migration of cranial neural crest cells (CNCCs) were unaffected. Mechanistically, the impaired PA region of nup62l-mutants underwent extensive apoptosis, which was mainly dependent on activation of p53-dependent apoptotic pathway. Moreover, aberrant activation of a series of apoptotic pathways in nup62l-mutants is closely associated with the inactivation of Wnt/β-catenin signaling. Thus, these findings suggest that the regulation of Wnt/β-catenin activity by Nup62l is crucial for PA formation in zebrafish.
Keywords: Nup62l; Wnt/β-catenin signaling; apoptosis; craniofacial development; pharyngeal arches.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Nucleoporin 62-like protein activates canonical Wnt signaling through facilitating the nuclear import of β-catenin in zebrafish.Mol Cell Biol. 2015 Apr;35(7):1110-24. doi: 10.1128/MCB.01181-14. Epub 2015 Jan 20. Mol Cell Biol. 2015. PMID: 25605329 Free PMC article.
-
Wnt2bb signaling promotes pharyngeal chondrogenic precursor proliferation and chondrocyte maturation by activating Yap expression in zebrafish.J Genet Genomics. 2025 Feb;52(2):220-230. doi: 10.1016/j.jgg.2024.11.006. Epub 2024 Nov 19. J Genet Genomics. 2025. PMID: 39566725
-
PRDM paralogs antagonistically balance Wnt/β-catenin activity during craniofacial chondrocyte differentiation.Development. 2022 Feb 15;149(4):dev200082. doi: 10.1242/dev.200082. Epub 2022 Feb 24. Development. 2022. PMID: 35132438 Free PMC article.
-
Context-dependent regulation of the β-catenin transcriptional complex supports diverse functions of Wnt/β-catenin signaling.J Biochem. 2017 Jan;161(1):9-17. doi: 10.1093/jb/mvw072. Epub 2016 Dec 24. J Biochem. 2017. PMID: 28013224 Review.
-
[Regulation of osteoblasts and chondrocytes by Wnt signaling.].Clin Calcium. 2019;29(3):299-307. doi: 10.20837/4201903299. Clin Calcium. 2019. PMID: 30814374 Review. Japanese.
Cited by
-
Role of Nucleoporins and Transport Receptors in Cell Differentiation.Front Physiol. 2020 Apr 3;11:239. doi: 10.3389/fphys.2020.00239. eCollection 2020. Front Physiol. 2020. PMID: 32308628 Free PMC article. Review.
-
New Activities of the Nuclear Pore Complexes.Cells. 2021 Aug 18;10(8):2123. doi: 10.3390/cells10082123. Cells. 2021. PMID: 34440892 Free PMC article.
-
Cornerstone Cellular Pathways for Metabolic Disorders and Diabetes Mellitus: Non-Coding RNAs, Wnt Signaling, and AMPK.Cells. 2023 Nov 9;12(22):2595. doi: 10.3390/cells12222595. Cells. 2023. PMID: 37998330 Free PMC article. Review.
-
Nuclear envelope and chromatin choreography direct cellular differentiation.Nucleus. 2025 Dec;16(1):2449520. doi: 10.1080/19491034.2024.2449520. Epub 2025 Feb 12. Nucleus. 2025. PMID: 39943681 Free PMC article. Review.
-
Functions of SMC2 in the Development of Zebrafish Liver.Biomedicines. 2021 Sep 16;9(9):1240. doi: 10.3390/biomedicines9091240. Biomedicines. 2021. PMID: 34572426 Free PMC article.
References
-
- Schilling T.F., Kimmel C.B. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development. 1994;120:483–494. - PubMed
-
- Kimmel C.B., Schilling T.F., Hatta K. Patterning of Body Segments of the Zebrafish Embryo. Curr. Top. Dev. Biol. 1991;25:77–110. - PubMed
-
- Noden D.M. Interactions and Fates of Avian Craniofacial Mesenchyme. Development. 1988;103:121–140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

