Differential Sympathetic Activation of Adipose Tissues by Brain-Derived Neurotrophic Factor
- PMID: 31492038
- PMCID: PMC6769916
- DOI: 10.3390/biom9090452
Differential Sympathetic Activation of Adipose Tissues by Brain-Derived Neurotrophic Factor
Abstract
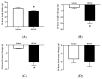
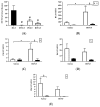
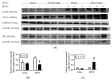
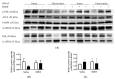
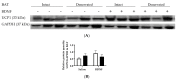
Centrally administered brain-derived neurotrophic factor (BDNF) decreases body adiposity beyond what can be accounted for by decreased food intake, implying enhanced lipid metabolism by BDNF. Consistent with this notion, intracerebroventricular (icv) injection of BDNF in rats increased the expression of lipolytic enzymes in white adipose tissues (WAT) and increased circulating concentrations of lipolytic products without changing the levels of adrenal gland hormones. This suggests that central BDNF-induced lipid mobilization is likely due to sympathetic neural activation, rather than activation of the adrenocortical or adrenomedullary system. We hypothesized that BDNF activated sympathetic innervation of adipose tissues to regulate lipolysis. Rats with unilateral denervation of interscapular brown adipose tissue (BAT) and different WAT depots received icv injections of saline or BDNF. Both intact and denervated adipose tissues were exposed to the same circulating factors, but denervated adipose tissues did not receive neural signals. Norepinephrine (NE) turnover (NETO) of BAT and WAT was assessed as a measure of sympathetic activity. Findings revealed that central BDNF treatment induced a change in NETO in some but not all the adipose tissues tested. Specifically, greater NETO rates were found in BAT and gonadal epididymal WAT (EWAT), but not in inguinal WAT (IWAT) or retroperitoneal WAT (RWAT), of BDNF-treated rats compared to saline-treated rats. Furthermore, intact innervation was necessary for BDNF-induced NETO in BAT and EWAT. In addition, BDNF increased the expression of lipolytic enzymes in both intact and denervated EWAT and IWAT, suggesting that BDNF-induced WAT lipolysis was independent of intact innervation. To summarize, centrally administered BDNF selectively provoked sympathetic drives to BAT and EWAT that was dependent on intact innervation, while BDNF also increased lipolysis in a manner independent of intact innervation.
Keywords: adrenal hormones; brown adipose tissue; denervation; energy homeostasis; lipolysis; sympathetic activity; white adipose tissue.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures






Similar articles
-
Sensory denervation of inguinal white fat modifies sympathetic outflow to white and brown fat in Siberian hamsters.Physiol Behav. 2018 Jun 1;190:28-33. doi: 10.1016/j.physbeh.2018.02.019. Epub 2018 Feb 12. Physiol Behav. 2018. PMID: 29447836 Free PMC article.
-
Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation.Endocrinology. 2007 Nov;148(11):5339-47. doi: 10.1210/en.2007-0621. Epub 2007 Aug 16. Endocrinology. 2007. PMID: 17702843
-
Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues.Am J Physiol Regul Integr Comp Physiol. 2010 Jul;299(1):R140-9. doi: 10.1152/ajpregu.00535.2009. Epub 2010 Apr 21. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20410474 Free PMC article.
-
Neural innervation of white adipose tissue and the control of lipolysis.Front Neuroendocrinol. 2014 Oct;35(4):473-93. doi: 10.1016/j.yfrne.2014.04.001. Epub 2014 Apr 13. Front Neuroendocrinol. 2014. PMID: 24736043 Free PMC article. Review.
-
Sensory and sympathetic nervous system control of white adipose tissue lipolysis.Mol Cell Endocrinol. 2010 Apr 29;318(1-2):34-43. doi: 10.1016/j.mce.2009.08.031. Epub 2009 Sep 10. Mol Cell Endocrinol. 2010. PMID: 19747957 Free PMC article. Review.
Cited by
-
Apolipoprotein A-IV Enhances Fatty Acid Uptake by Adipose Tissues of Male Mice via Sympathetic Activation.Endocrinology. 2020 Apr 1;161(4):bqaa042. doi: 10.1210/endocr/bqaa042. Endocrinology. 2020. PMID: 32157301 Free PMC article.
-
Amino Acid-Sensing Neurons in the Anterior Piriform Cortex Control Brown Adipose Tissue Thermogenesis.Adv Sci (Weinh). 2025 Jul;12(27):e2502421. doi: 10.1002/advs.202502421. Epub 2025 Apr 30. Adv Sci (Weinh). 2025. PMID: 40305738 Free PMC article.
-
Metabolic protection by the dietary flavonoid 7,8-dihydroxyflavone requires an intact gut microbiome.Front Nutr. 2022 Aug 17;9:987956. doi: 10.3389/fnut.2022.987956. eCollection 2022. Front Nutr. 2022. PMID: 36061902 Free PMC article.
-
Physical Exercise-Induced Myokines in Neurodegenerative Diseases.Int J Mol Sci. 2021 May 28;22(11):5795. doi: 10.3390/ijms22115795. Int J Mol Sci. 2021. PMID: 34071457 Free PMC article. Review.
-
Comparative Transcriptome Profile Analysis of Longissimus dorsi Muscle Tissues From Two Goat Breeds With Different Meat Production Performance Using RNA-Seq.Front Genet. 2021 Jan 13;11:619399. doi: 10.3389/fgene.2020.619399. eCollection 2020. Front Genet. 2021. PMID: 33519920 Free PMC article.
References
-
- Toriya M., Maekawa F., Maejima Y., Onaka T., Fujiwara K., Nakagawa T., Nakata M., Yada T. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 2010;22:987–995. doi: 10.1111/j.1365-2826.2010.02039.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

