Phase Separation-Mediated TARP/MAGUK Complex Condensation and AMPA Receptor Synaptic Transmission
- PMID: 31492534
- PMCID: PMC6842113
- DOI: 10.1016/j.neuron.2019.08.001
Phase Separation-Mediated TARP/MAGUK Complex Condensation and AMPA Receptor Synaptic Transmission
Abstract
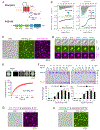
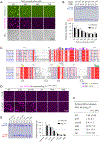
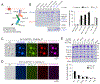
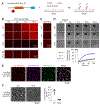
Transmembrane AMPA receptor (AMPAR) regulatory proteins (TARPs) modulate AMPAR synaptic trafficking and transmission via disc-large (DLG) subfamily of membrane-associated guanylate kinases (MAGUKs). Despite extensive studies, the molecular mechanism governing specific TARP/MAGUK interaction remains elusive. Using stargazin and PSD-95 as the representatives, we discover that the entire tail of stargazin (Stg_CT) is required for binding to PSD-95. The PDZ binding motif (PBM) and an Arg-rich motif upstream of PBM conserved in TARPs bind to multiple sites on PSD-95, thus resulting in a highly specific and multivalent stargazin/PSD-95 complex. Stargazin in complex with PSD-95 or PSD-95-assembled postsynaptic complexes form highly concentrated and dynamic condensates via phase separation, reminiscent of stargazin/PSD-95-mediated AMPAR synaptic clustering and trapping. Importantly, charge neutralization mutations in TARP_CT Arg-rich motif weakened TARP's condensation with PSD-95 and impaired TARP-mediated AMPAR synaptic transmission in mice hippocampal neurons. The TARP_CT/PSD-95 interaction mode may have implications for understanding clustering of other synaptic transmembrane proteins.
Keywords: AMPAR; MAGUK; PSD-95; TARP; biological condensates; phase separation; postsynaptic density; stargazin; synaptic transmission.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures



Similar articles
-
The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking.Neuron. 2007 Mar 1;53(5):719-34. doi: 10.1016/j.neuron.2007.01.030. Neuron. 2007. PMID: 17329211
-
TARP γ-2 and γ-8 Differentially Control AMPAR Density Across Schaffer Collateral/Commissural Synapses in the Hippocampal CA1 Area.J Neurosci. 2016 Apr 13;36(15):4296-312. doi: 10.1523/JNEUROSCI.4178-15.2016. J Neurosci. 2016. PMID: 27076426 Free PMC article.
-
Stargazin and other transmembrane AMPA receptor regulating proteins interact with synaptic scaffolding protein MAGI-2 in brain.J Neurosci. 2006 Jul 26;26(30):7875-84. doi: 10.1523/JNEUROSCI.1851-06.2006. J Neurosci. 2006. PMID: 16870733 Free PMC article.
-
Transmembrane AMPA receptor regulatory proteins and AMPA receptor function in the cerebellum.Neuroscience. 2009 Sep 1;162(3):656-65. doi: 10.1016/j.neuroscience.2009.01.004. Epub 2009 Jan 13. Neuroscience. 2009. PMID: 19185052 Free PMC article. Review.
-
The role of transmembrane AMPA receptor regulatory proteins (TARPs) in neurotransmission and receptor trafficking (Review).Mol Membr Biol. 2008 May;25(4):353-62. doi: 10.1080/09687680801986480. Mol Membr Biol. 2008. PMID: 18446621 Review.
Cited by
-
Molecular physiology of Arc/Arg3.1: The oligomeric state hypothesis of synaptic plasticity.Acta Physiol (Oxf). 2022 Nov;236(3):e13886. doi: 10.1111/apha.13886. Epub 2022 Sep 20. Acta Physiol (Oxf). 2022. PMID: 36073248 Free PMC article. Review.
-
Remembering and forgetting in sleep: Selective synaptic plasticity during sleep driven by scaling factors Homer1a and Arc.Neurobiol Stress. 2022 Dec 31;22:100512. doi: 10.1016/j.ynstr.2022.100512. eCollection 2023 Jan. Neurobiol Stress. 2022. PMID: 36632309 Free PMC article.
-
Correlative Assembly of Subsynaptic Nanoscale Organizations During Development.Front Synaptic Neurosci. 2022 May 24;14:748184. doi: 10.3389/fnsyn.2022.748184. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35685244 Free PMC article.
-
Twenty Years of SynGAP Research: From Synapses to Cognition.J Neurosci. 2020 Feb 19;40(8):1596-1605. doi: 10.1523/JNEUROSCI.0420-19.2020. J Neurosci. 2020. PMID: 32075947 Free PMC article. Review.
-
AMPA receptor diffusional trapping machinery as an early therapeutic target in neurodegenerative and neuropsychiatric disorders.Transl Neurodegener. 2025 Feb 11;14(1):8. doi: 10.1186/s40035-025-00470-z. Transl Neurodegener. 2025. PMID: 39934896 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

