Mammalian kinetochores count attached microtubules in a sensitive and switch-like manner
- PMID: 31492713
- PMCID: PMC6829666
- DOI: 10.1083/jcb.201902105
Mammalian kinetochores count attached microtubules in a sensitive and switch-like manner
Abstract
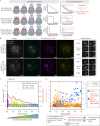
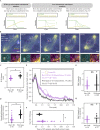
The spindle assembly checkpoint (SAC) prevents anaphase until all kinetochores attach to the spindle. Each mammalian kinetochore binds many microtubules, but how many attached microtubules are required to turn off the checkpoint, and how the kinetochore monitors microtubule numbers, are not known and are central to understanding SAC mechanisms and function. To address these questions, here we systematically tune and fix the fraction of Hec1 molecules capable of microtubule binding. We show that Hec1 molecules independently bind microtubules within single kinetochores, but that the kinetochore does not independently process attachment information from different molecules. Few attached microtubules (20% occupancy) can trigger complete Mad1 loss, and Mad1 loss is slower in this case. Finally, we show using laser ablation that individual kinetochores detect changes in microtubule binding, not in spindle forces that accompany attachment. Thus, the mammalian kinetochore responds specifically to the binding of each microtubule and counts microtubules as a single unit in a sensitive and switch-like manner. This may allow kinetochores to rapidly react to early attachments and maintain a robust SAC response despite dynamic microtubule numbers.
© 2019 Kuhn and Dumont.
Figures



Similar articles
-
Kinetochore-microtubule attachment is sufficient to satisfy the human spindle assembly checkpoint.Nat Commun. 2015 Dec 1;6:8987. doi: 10.1038/ncomms9987. Nat Commun. 2015. PMID: 26621779 Free PMC article.
-
Stable kinetochore-microtubule attachment is sufficient to silence the spindle assembly checkpoint in human cells.Nat Commun. 2015 Dec 1;6:10036. doi: 10.1038/ncomms10036. Nat Commun. 2015. PMID: 26620470 Free PMC article.
-
Spindle assembly checkpoint satisfaction occurs via end-on but not lateral attachments under tension.J Cell Biol. 2017 Jun 5;216(6):1533-1542. doi: 10.1083/jcb.201611104. Epub 2017 May 23. J Cell Biol. 2017. PMID: 28536121 Free PMC article.
-
Merotelic kinetochores in mammalian tissue cells.Philos Trans R Soc Lond B Biol Sci. 2005 Mar 29;360(1455):553-68. doi: 10.1098/rstb.2004.1610. Philos Trans R Soc Lond B Biol Sci. 2005. PMID: 15897180 Free PMC article. Review.
-
Dynamic regulation of kinetochore-microtubule interaction during mitosis.J Biochem. 2012 Nov;152(5):415-24. doi: 10.1093/jb/mvs109. Epub 2012 Sep 20. J Biochem. 2012. PMID: 22995986 Review.
Cited by
-
Evaluation of the Spindle Assembly Checkpoint Integrity in Mouse Oocytes.J Vis Exp. 2022 Sep 13;(187):10.3791/64459. doi: 10.3791/64459. J Vis Exp. 2022. PMID: 36190266 Free PMC article.
-
Non-autonomous insulin signaling delays mitotic progression in C. elegans germline stem and progenitor cells.PLoS Genet. 2024 Dec 23;20(12):e1011351. doi: 10.1371/journal.pgen.1011351. eCollection 2024 Dec. PLoS Genet. 2024. PMID: 39715269 Free PMC article.
-
Hec1/Ndc80 Tail Domain Function at the Kinetochore-Microtubule Interface.Front Cell Dev Biol. 2020 Feb 26;8:43. doi: 10.3389/fcell.2020.00043. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32161753 Free PMC article. Review.
-
Structure of the RZZ complex and molecular basis of Spindly-driven corona assembly at human kinetochores.EMBO J. 2022 May 2;41(9):e110411. doi: 10.15252/embj.2021110411. Epub 2022 Apr 4. EMBO J. 2022. PMID: 35373361 Free PMC article.
-
The Hec1/Ndc80 tail domain is required for force generation at kinetochores, but is dispensable for kinetochore-microtubule attachment formation and Ska complex recruitment.Mol Biol Cell. 2020 Jul 1;31(14):1453-1473. doi: 10.1091/mbc.E20-05-0286. Epub 2020 May 13. Mol Biol Cell. 2020. PMID: 32401635 Free PMC article.

