Low-dose ladostigil for mild cognitive impairment: A phase 2 placebo-controlled clinical trial
- PMID: 31492718
- PMCID: PMC7010322
- DOI: 10.1212/WNL.0000000000008239
Low-dose ladostigil for mild cognitive impairment: A phase 2 placebo-controlled clinical trial
Abstract
Objective: Ladostigil reduces oxidative stress and microglial activation in aging rats. We assessed its safety and potential efficacy in a 3-year, randomized, double-blind, placebo-controlled phase 2 clinical trial in patients with mild cognitive impairment (MCI) and medial temporal lobe atrophy.
Methods: Patients 55 to 85 years of age with MCI, Clinical Dementia Rating (CDR) score of 0.5, Mini-Mental State Examination (MMSE) score >24, Wechsler Memory Scale-Revised Verbal Paired Associates I score ≤18, and Medial Temporal Lobe Atrophy Scale score >1 were stratified by APOE ε4 genotype and randomly assigned (1:1) to ladostigil 10 mg/d or placebo. Primary outcomes were safety and onset of Alzheimer disease dementia. Secondary endpoints were Neuropsychological Test Battery (NTB) composite, Disability Assessment in Dementia (DAD), and Geriatric Depression Scale (GDS) scores. Exploratory outcomes were NTB component, CDR, and MMSE scores. Biomarkers included MRI-derived whole-brain, hippocampus, and entorhinal cortex volumes.
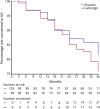
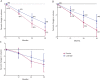
Results: Two hundred ten patients from 15 sites in Austria, Germany, and Israel were randomly allocated to placebo (107 patients) or ladostigil (103 patients). After 36 months, 21 of 103 patients on placebo and 14 of 99 patients receiving ladostigil progressed to Alzheimer disease (log-rank test p = 0.162). There were no significant effects on the NTB composite, DAD, or GDS score. Whole-brain and hippocampus volumes decreased more in the placebo than in the ladostigil group (whole brain, p = 0.025, Cohen d = 0.43; hippocampus, p = 0.043, d = 0.43). Serious adverse events were reported by 28 of 107 patients treated with placebo and 26 of 103 with ladostigil.
Conclusion: Ladostigil was safe and well tolerated but did not delay progression to dementia. Its association with reduced brain and hippocampus volume loss suggests a potential effect on atrophy.
Clinicaltrialsgov identifier: NCT01429623.
Classification of evidence: This study provides Class II evidence that for patients with MCI and medial temporal lobe atrophy, ladostigil did not significantly decrease the risk of the development of Alzheimer disease.
© 2019 American Academy of Neurology.
Figures

References
-
- Weinstock M, Gorodetsky E, Poltyrev T, Gross A, Sagi Y, Youdim M. A novel cholinesterase and brain-selective monoamine oxidase inhibitor for the treatment of dementia comorbid with depression and Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:555–561. - PubMed
-
- Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563–572. - PubMed
-
- Maruyama W, Weinstock M, Youdim MB, Nagai M, Naoi M. Anti-apoptotic action of anti-Alzheimer drug, TV3326 [(N-propargyl)-(3R)-aminoindan-5-yl]-ethyl methyl carbamate, a novel cholinesterase-monoamine oxidase inhibitor. Neurosci Lett 2003;341:233–236. - PubMed
-
- Weinstock M, Luques L, Poltyrev T, Bejar C, Shoham S. Ladostigil prevents age-related glial activation and spatial memory deficits in rats. Neurobiol Aging 2011;32:1069–1078. - PubMed
-
- Panarsky R, Luques L, Weinstock M. Anti-inflammatory effects of ladostigil and its metabolites in aged rat brain and in microglial cells. J Neuroimmune Pharmacol 2012;7:488–498. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
