Cytosolic PCNA interacts with p47phox and controls NADPH oxidase NOX2 activation in neutrophils
- PMID: 31492810
- PMCID: PMC6829599
- DOI: 10.1084/jem.20180371
Cytosolic PCNA interacts with p47phox and controls NADPH oxidase NOX2 activation in neutrophils
Erratum in
-
Correction: Cytosolic PCNA interacts with p47phox and controls NADPH oxidase NOX2 activation in neutrophils.J Exp Med. 2019 Dec 2;216(12):2900. doi: 10.1084/jem.2018037111082019c. Epub 2019 Nov 15. J Exp Med. 2019. PMID: 31732507 Free PMC article. No abstract available.
Abstract
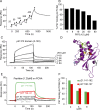
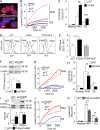
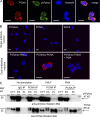
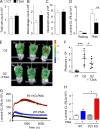
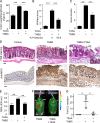
Neutrophils produce high levels of reactive oxygen species (ROS) by NADPH oxidase that are crucial for host defense but can lead to tissue injury when produced in excess. We previously described that proliferating cell nuclear antigen (PCNA), a nuclear scaffolding protein pivotal in DNA synthesis, controls neutrophil survival through its cytosolic association with procaspases. We herein showed that PCNA associated with p47phox, a key subunit of NADPH oxidase, and that this association regulated ROS production. Surface plasmon resonance and crystallography techniques demonstrated that the interdomain-connecting loop of PCNA interacted directly with the phox homology (PX) domain of the p47phox. PCNA inhibition by competing peptides or by T2AA, a small-molecule PCNA inhibitor, decreased NADPH oxidase activation in vitro. Furthermore, T2AA provided a therapeutic benefit in mice during trinitro-benzene-sulfonic acid (TNBS)-induced colitis by decreasing oxidative stress, accelerating mucosal repair, and promoting the resolution of inflammation. Our data suggest that targeting PCNA in inflammatory neutrophils holds promise as a multifaceted antiinflammatory strategy.
© 2019 Ohayon et al.
Figures



References
-
- Ameho C.K., Adjei A.A., Harrison E.K., Takeshita K., Morioka T., Arakaki Y., Ito E., Suzuki I., Kulkarni A.D., Kawajiri A., and Yamamoto S.. 1997. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 41:487–493. 10.1136/gut.41.4.487 - DOI - PMC - PubMed
-
- Baillet A., Hograindleur M.A., El Benna J., Grichine A., Berthier S., Morel F., and Paclet M.H.. 2017. Unexpected function of the phagocyte NADPH oxidase in supporting hyperglycolysis in stimulated neutrophils: key role of 6-phosphofructo-2-kinase. FASEB J. 31:663–673. 10.1096/fj.201600720R - DOI - PubMed
-
- Boehm E.M., and Washington M.T.. 2016. R.I.P. to the PIP: PCNA-binding motif no longer considered specific: PIP motifs and other related sequences are not distinct entities and can bind multiple proteins involved in genome maintenance. BioEssays. 38:1117–1122. 10.1002/bies.201600116 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

