An ensemble of cryo-EM structures of TRiC reveal its conformational landscape and subunit specificity
- PMID: 31492816
- PMCID: PMC6765261
- DOI: 10.1073/pnas.1903976116
An ensemble of cryo-EM structures of TRiC reveal its conformational landscape and subunit specificity
Abstract
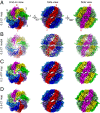
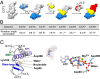
TRiC/CCT assists the folding of ∼10% of cytosolic proteins through an ATP-driven conformational cycle and is essential in maintaining protein homeostasis. Here, we determined an ensemble of cryo-electron microscopy (cryo-EM) structures of yeast TRiC at various nucleotide concentrations, with 4 open-state maps resolved at near-atomic resolutions, and a closed-state map at atomic resolution, revealing an extra layer of an unforeseen N-terminal allosteric network. We found that, during TRiC ring closure, the CCT7 subunit moves first, responding to nucleotide binding; CCT4 is the last to bind ATP, serving as an ATP sensor; and CCT8 remains ADP-bound and is hardly involved in the ATPase-cycle in our experimental conditions; overall, yeast TRiC consumes nucleotide in a 2-ring positively coordinated manner. Our results depict a thorough picture of the TRiC conformational landscape and its allosteric transitions from the open to closed states in more structural detail and offer insights into TRiC subunit specificity in ATP consumption and ring closure, and potentially in substrate processing.
Keywords: ATPase cycle; allosteric network; chaperonin TRiC/CCT; conformational landscape; cryo-EM.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Staggered ATP binding mechanism of eukaryotic chaperonin TRiC (CCT) revealed through high-resolution cryo-EM.Nat Struct Mol Biol. 2016 Dec;23(12):1083-1091. doi: 10.1038/nsmb.3309. Epub 2016 Oct 24. Nat Struct Mol Biol. 2016. PMID: 27775711
-
State-dependent sequential allostery exhibited by chaperonin TRiC/CCT revealed by network analysis of Cryo-EM maps.Prog Biophys Mol Biol. 2021 Mar;160:104-120. doi: 10.1016/j.pbiomolbio.2020.08.006. Epub 2020 Aug 28. Prog Biophys Mol Biol. 2021. PMID: 32866476 Free PMC article.
-
Sequential allosteric mechanism of ATP hydrolysis by the CCT/TRiC chaperone is revealed through Arrhenius analysis.Proc Natl Acad Sci U S A. 2017 May 16;114(20):5189-5194. doi: 10.1073/pnas.1617746114. Epub 2017 May 1. Proc Natl Acad Sci U S A. 2017. PMID: 28461478 Free PMC article.
-
TRiC/CCT Chaperonin: Structure and Function.Subcell Biochem. 2019;93:625-654. doi: 10.1007/978-3-030-28151-9_19. Subcell Biochem. 2019. PMID: 31939165 Review.
-
The ATP-powered gymnastics of TRiC/CCT: an asymmetric protein folding machine with a symmetric origin story.Curr Opin Struct Biol. 2019 Apr;55:50-58. doi: 10.1016/j.sbi.2019.03.002. Epub 2019 Apr 9. Curr Opin Struct Biol. 2019. PMID: 30978594 Free PMC article. Review.
Cited by
-
Intrinsic dynamics is evolutionarily optimized to enable allosteric behavior.Curr Opin Struct Biol. 2020 Jun;62:14-21. doi: 10.1016/j.sbi.2019.11.002. Epub 2019 Nov 27. Curr Opin Struct Biol. 2020. PMID: 31785465 Free PMC article. Review.
-
Single-molecule visualization determines conformational substate ensembles in β-sheet-rich peptide fibrils.Sci Adv. 2023 Jul 7;9(27):eadg7943. doi: 10.1126/sciadv.adg7943. Epub 2023 Jul 5. Sci Adv. 2023. PMID: 37406110 Free PMC article.
-
A structural vista of phosducin-like PhLP2A-chaperonin TRiC cooperation during the ATP-driven folding cycle.Nat Commun. 2024 Feb 2;15(1):1007. doi: 10.1038/s41467-024-45242-x. Nat Commun. 2024. PMID: 38307855 Free PMC article.
-
CryoEM structural exploration of catalytically active enzyme pyruvate carboxylase.Nat Commun. 2022 Oct 19;13(1):6185. doi: 10.1038/s41467-022-33987-2. Nat Commun. 2022. PMID: 36261450 Free PMC article.
-
Structural basis of substrate progression through the bacterial chaperonin cycle.Proc Natl Acad Sci U S A. 2023 Dec 12;120(50):e2308933120. doi: 10.1073/pnas.2308933120. Epub 2023 Dec 8. Proc Natl Acad Sci U S A. 2023. PMID: 38064510 Free PMC article.
References
-
- Llorca O., et al. , Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature 402, 693–696 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

