Continuous artificial synthesis of glucose precursor using enzyme-immobilized microfluidic reactors
- PMID: 31492867
- PMCID: PMC6731257
- DOI: 10.1038/s41467-019-12089-6
Continuous artificial synthesis of glucose precursor using enzyme-immobilized microfluidic reactors
Abstract
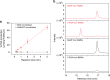
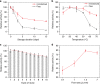
Food production in green crops is severely limited by low activity and poor specificity of D-ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) in natural photosynthesis (NPS). This work presents a scientific solution to overcome this problem by immobilizing RuBisCO into a microfluidic reactor, which demonstrates a continuous production of glucose precursor at 13.8 μmol g-1 RuBisCO min-1 from CO2 and ribulose-1,5-bisphosphate. Experiments show that the RuBisCO immobilization significantly enhances enzyme stabilities (7.2 folds in storage stability, 6.7 folds in thermal stability), and also improves the reusability (90.4% activity retained after 5 cycles of reuse and 78.5% after 10 cycles). This work mimics the NPS pathway with scalable microreactors for continuous synthesis of glucose precursor using very small amount of RuBisCO. Although still far from industrial production, this work demonstrates artificial synthesis of basic food materials by replicating the light-independent reactions of NPS, which may hold the key to food crisis relief and future space colonization.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Berg, J. M., Tymoczko, J. L. & Stryer, L. Biochemistry, 7th edn (W. H. Freeman, 2012).
-
- Archer, M. D. & Barber, J. Molecular to Global Photosynthesis (World Scientific, 2004).
-
- Northoff, E. 2050 A Third More Mouths to Feed (Food and Agriculture Organization of the United Nations, 2016).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

