Novel Preparations of Glucagon for the Prevention and Treatment of Hypoglycemia
- PMID: 31493043
- PMCID: PMC6951434
- DOI: 10.1007/s11892-019-1216-4
Novel Preparations of Glucagon for the Prevention and Treatment of Hypoglycemia
Abstract
Purpose of review: New more stable formulations of glucagon have recently become available, and these provide an opportunity to expand the clinical roles of this hormone in the prevention and management of insulin-induced hypoglycemia. This is applicable in type 1 diabetes, hyperinsulinism, and alimentary hypoglycemia. The aim of this review is to describe these new formulations of glucagon and to provide an overview of current and future therapeutic opportunities that these may provide.
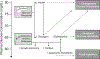
Recent findings: Four main categories of glucagon formulation have been studied: intranasal glucagon, biochaperone glucagon, dasiglucagon, and non-aqueous soluble glucagon. All four have demonstrated similar glycemic responses to standard glucagon formulations when administered during hypoglycemia. In addition, potential roles of these formulations in the management of congenital hyperinsulinism, alimentary hypoglycemia, and exercise-induced hypoglycemia in type 1 diabetes have been described. As our experience with newer glucagon preparations increases, the role of glucagon is likely to expand beyond the emergency use that this medication has been limited to in the past. The innovations described in this review likely represent early examples of a pending large repertoire of indications for stable glucagon.
Keywords: Alimentary; Diabetes; Formulation; Glucagon; Hyperinsulinism; Hypoglycemia.
Conflict of interest statement
Figures
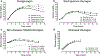
References
-
- Hawkes CP, Lado JJ, Givler S, De Leon DD. The effect of continuous intravenous glucagon on glucose requirements in infants with congenital hyperinsulinism. JIMD rep. 2019;45:45–50. 10.1007/8904_2018_140. - DOI - PMC - PubMed
-
• This study demonstrates the effect of a continuous intravenous glucagon infusion on glucose requirement on infants with congenital hyperinsulinism.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

