Phenotypic characteristics of human bone marrow-derived endothelial progenitor cells in vitro support cell effectiveness for repair of the blood-spinal cord barrier in ALS
- PMID: 31493389
- PMCID: PMC6779528
- DOI: 10.1016/j.brainres.2019.146428
Phenotypic characteristics of human bone marrow-derived endothelial progenitor cells in vitro support cell effectiveness for repair of the blood-spinal cord barrier in ALS
Abstract
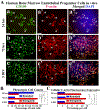
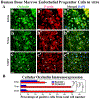
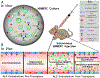
Amyotrophic lateral sclerosis (ALS) was recently recognized as a neurovascular disease. Accumulating evidence demonstrated blood-spinal-cord barrier (BSCB) impairment mainly via endothelial cell (EC) degeneration in ALS patients and animal models. BSCB repair may be a therapeutic approach for ALS. We showed benefits of human bone marrow endothelial progenitor cell (hBMEPC) transplantation into symptomatic ALS mice on barrier restoration; however, cellular mechanisms remain unclear. The study aimed to characterize hBMEPCs in vitro under normogenic conditions. hBMEPCs were cultured at different time points. Enzyme-linked immunosorbent assay (ELISA) was used to detect concentrations of angiogenic factors (VEGF-A, angiogenin-1, and endoglin) and angiogenic inhibitor endostatin in conditioned media. Double immunocytochemical staining for CD105, ZO-1, and occludin with F-actin was performed. Results showed predominantly gradual significant post-culture increases of VEGF-A and angiogenin-1 levels. Cultured cells displayed distinct rounded or elongated cellular morphologies and positively immunoexpressed for CD105, indicating EC phenotype. Cytoskeletal F-actin filaments were re-arranged according to cell morphologies. Immunopositive expressions for ZO-1 were detected near inner cell membrane and for occludin on cell membrane surface of adjacent hBMEPCs. Together, secretion of angiogenic factors by cultured cells provides evidence for a potential mechanism underlying endogenous EC repair in ALS through hBMEPC transplantation, leading to restored barrier integrity. Also, ZO-1 and occludin immunoexpressions, confirming hBMEPC interactions in vitro, may reflect post-transplant cell actions in vivo.
Keywords: Angiogenic factors; F-actin; Human bone marrow endothelial progenitor cells; In vitro; Tight junction proteins.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

